Category: Critical Care
Posted: 5/15/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Balloon Tamponade for Variceal Bleeding
Category: Critical Care
Posted: 5/8/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Severe acute pancreatitis (SAP) is a life-threatening form of pancreatitis, with up to 30% mortality.
SAP may lead to hypovolemic shock (secondary to vasodilation and capillary leak), hypoxemia (from acute respiratory distress syndrome), and multi-organ failure.
Suspect SAP with signs and symptoms of pancreatitis plus any of the following:
Treatment of SAP should focus on:
Greer, S. E., & Burchard, K. W. (2009). Acute pancreatitis and critical illness: a pancreatic tale of hypoperfusion and inflammation. Chest, 136(5), 1413–1419.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Keywords: spontaenous bacterial peritonitis, hepatorenal syndrome, albumin (PubMed Search)
Posted: 5/1/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
SBP, HRS, and Albumin
Bernardi M, et al. Human albumin in the management of complications of liver cirrhosis. Crit Care 2012; 16:211.
Category: Critical Care
Posted: 4/24/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
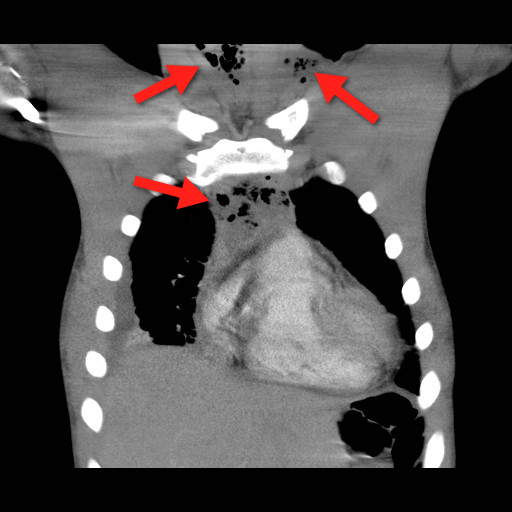
Mediastinitis is an infection of the mediastinum; a rapidly fatal surgical emergency if not recognized and treated early.
Causes include esophageal perforation, oropharyngeal infections (e.g., Ludwig’s angina), prevertebral or carotid space infections, and iatrogenically (endoscopy, hypopharyngeal perforations during intubation, etc.).
Plain films (neck / chest) may serve as a screening tool, but CT best defines the source and extent of disease; the CT below demonstrates gas within the soft-tissues and the mediastinum (red arrrows).
Infections may be polymicrobial and broad-spectrum antibiotics with anaerobic coverage (e.g., pipercillin-tazobacam) should be started initially.
Immediate treatment should also include:
Bonus Pearl
Can't keep up with all the great educational stuff in Emergency Medicine and Critical Care? Let the professionals at Life in the Fastlane do it for you (http://lifeinthefastlane.com). These guys scour the web and blog about the best educational pearls, podcasts, and radoiolgic finds...and they're also quite the laugh. Check them out today!
Ridder G, et al. Descending necrotizing mediastinitis: contemporary trends in etiology, diagnosis, management, and outcome. Ann Surg. Mar 2010;251(3):528-34.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 4/17/2012 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Cuff Pressures and the Prevention of VAP
Grap MJ, et al. Ventilator-associated pneumonia: The potential critical role of emergency medicine in prevention. JEM 2012; 42:353-362.
Category: Critical Care
Posted: 4/9/2012 by Haney Mallemat, MD
(Updated: 8/12/2014)
Click here to contact Haney Mallemat, MD
Sepsis is one of the top 10 causes of death in the U.S. and its incidence is on the rise.
The financial burden of sepsis is also growing; it is estimated that between 2000 and 2005 the overall cost of ICU hospitalization rose from $56.6 billion to $81.7 billion per year with severe sepsis accounting for $16.7 billion.
Although we may not be able to immediately modify the incidence of community-acquired sepsis, hospital-acquired sepsis can be reduced; for example, many cases of nosocomial sepsis are associated with catheter blood stream infections secondary to central-lines.
There are several simple strategies to prevent catheter-related blood-stream infections:
Remember: We play a large role in reducing nosocomial sepsis; be vigilant about your sterile techniques during central catheter insertions and question the need for every single line.
Bonus pearl (only for iPhone): MDRNTools is a FREE app (that’s right, FREE!) with lots of ED and ICU applications such as an IV med calculator, an RSI handbook, a Stroke Scale calculator, and more.
Download http://itunes.apple.com/us/app/mdrntools/id505794224?mt=8&ls=1
Chalupka, A. N., & Talmor, D. (2012). The Economics of Sepsis. CCC, 28(1), 57–76.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 4/3/2012 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Transferring Multidrug-Resistant Organisms
Morgan DJ, et al. Transfer of multidrug-resistant bacteria to healthcare workers' gloves and gowns after patient contact increases with environmental contamination. Crit Care Med 2012; 40:1045-1051.
Category: Critical Care
Keywords: apnea time, rapid sequence intubation, atelectasis, crticial care, intubation, hypoexemia, obesity (PubMed Search)
Posted: 3/26/2012 by Haney Mallemat, MD
(Updated: 3/27/2012)
Click here to contact Haney Mallemat, MD
The supine position during rapid sequence intubation may result in posterior lung atelectasis thereby reducing lung volumes, oxygenation reserve, and ultimately apnea time.
Several studies have shown that elevating the head of the bed by at least 20 degrees or placing a patient in reverse Trendelenberg position (for patients with contra-indications to elevating the head of the bed) during RSI may significantly increase apnea time.
Elevating the head of the bed may be especially helpful for patients with BMIs >35
Weingart, S and Levitan, R. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012 Mar; 59(3):165-175.e1; here's the article for FREE: http://www.annemergmed.com/article/S0196-0644(11)01667-2/fulltext
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 3/20/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
High-Frequency Oscillatory Ventilation for ARDS?
Ip T, Mehta S. The role of high-frequency oscillatory ventilation in the treatment of acute respiratory failure in adults. Curr Opin Crit Care 2012; 18:70-9.
Category: Critical Care
Posted: 3/13/2012 by Haney Mallemat, MD
(Updated: 3/14/2012)
Click here to contact Haney Mallemat, MD
Pre-oxygenation prior to rapid sequence intubation (RSI) is performed to prevent hypoxemia during endotracheal intubation.
An appropriate period of pre-oxygenation will potentially increase the amount of apnea time during intubation, however patients with certain critical illnesses (e.g., severe pneumonia) may desaturate faster than expected.
Apnea time can be increased by maintaining high-flow oxygen by nasal cannula (e.g., 15L), during application of the bag-valve mask and during the time of attempted endotracheal tube placement; this concept is known as apneic oxygenation.
Apneic oxygenation is based on the principle that when patients are apneic, alveoli absorb oxygen into the blood stream at a rate of approximately 250 mL/minute, creating a diffusion gradient from the pharynx (containing a high-density of oxygen from the nasal cannula) to a lower concentration of oxygen in the alveoli.
Although a patient’s oxygenation can be maintained longer using apneic oxygenation, its application does not remove the continuous buildup of CO2 in the alveoli during apena. Therefore, respiratory acidosis can result after a prolonged period of apneic oxygenation.
The complete article describing the physiology and practical applications can be found here....it's free! http://www.annemergmed.com/article/S0196-0644(11)01667-2/fulltext
Weingart, S and Levitan, R. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012 Mar; 59(3):165-175.e1
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 3/6/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Preventing VAP in the Intubated ED Patient
Ramirez P, et al. Measures to prevent nosocomial infections during mechanical ventilation. Curr Opin Crit Care 2012;18:86-92.
Category: Critical Care
Keywords: VAD, ventricular assist device, hear failure, shock, hemodynamics (PubMed Search)
Posted: 2/28/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Ventricular assist devices (VAD) pump blood from the left, right or both ventricles for patients in severe ventricular failure.
VADs may be placed temporarily (as a bridge to transplant) or permanently in patients who are not transplant candidates (also known as Destination Therapy)
Certain types of VADs continuously pump blood in a non-pulsatile fashion. In these cases, a patient may be perfusing normally without a palpable pulse.
Familiarity with potential VAD complications is important as a patient with a VAD may be presenting to an ED near you. Complications include:
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 2/21/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Ice-Cold Crystalloid for Therapeutic Hypothermia
Arulkumaran N, Ball SJ. Use of ice-cold crystalloid for inducing mild therapeutic hypothermia following out-of-hospital cardiac arrest. Resuscitation 2012;83:151-8.
Category: Critical Care
Keywords: pericardial tampaonde, shock, tamponade, fluids, hypoperfusion (PubMed Search)
Posted: 2/13/2012 by Haney Mallemat, MD
(Updated: 2/15/2012)
Click here to contact Haney Mallemat, MD
A fluid bolus is often the first-line therapy for patients with pericardial tamponade. A fluid bolus, however, may not always improve hemodynamics.
The cardiac index of forty-nine patients with cardiac tamponade was assessed before and after a 500 cc normal saline bolus:
Bottom-line: A fluid bolus may a reasonable first choice in a hypotensive patient with tamponade, but remember that fluid boluses may not always work. Attempts at fluid resuscitation should never delay definitive treatment with pericardiocentesis.
Sagrista-Sauleda, et al. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation 2008; 117:1545
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 2/7/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
ECMO for ARDS and Refractory Hypoxemia
Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS is adults. NEJM 2011;365:1905-14.
Category: Critical Care
Posted: 1/31/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
AGE occurs when gas bubbles enter arteries or veins; AGE may cause clinical symptoms even with very small volumes of air.
Air enters the circulatory system via:
· Barotrauma – Alveolar injury allows air to enter systemic bloodstream; occurs in divers following rapid ascent after breath holding, during mechanical ventilation, chest tube placement, or bronchoscopy
· Decompression sickness – Dissolved gas precipitates out of bloodstream as bubbles; typically following scuba diving without appropriate time to ascend or prolonged flying in unpressurized aircrafts
· Direct injection of air into arterial or venous circulation – Examples include accidental IV injection of air, needle biopsy of lung, or aspiration of air during central line placement
Serious clinical manifestations include:
· Neurologic changes - loss of consciousness, confusion, or focal neurological deficits
· Hemodynamic changes – hypotension, arrhythmias, cardiac ischemia, or cardiac arrest.
· Respiratory changes – obstruction of pulmonary circulation, pulmonary edema, or hypoxemia
Treatment:
· Strict attention to ABC’s using high-flow O2.
· Keep head of bed elevated to minimize/reduce cerebral edema.
· Hyperbaric Oxygen (HBO) therapy is recommended for neurological manifestations or cardiovascular instability. Good outcomes associated with shorter intervals from air embolism to HBO. Typically only 1 to 2 treatments are needed; occasionally additional treatments are necessary.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 1/24/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
SAH and Pulmonary Edema - Think Twice About Diuresis!
Scalfani MT, Diringer MN. Year in review 2010: Critical Care - neurocritical care. Crit Care 2011;15:237.
Category: Critical Care
Keywords: fungal, endopthalmitis, ocular, critically ill, systemic infection, immunosupression, IVDA (PubMed Search)
Posted: 1/17/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Fungal endopthalmitis is an intraocular infection of the aqueous and/or vitreous humor secondary to fungal pathogens; Candida and Aspergillus species are the most common pathogens.
Risk factors: intravenous drug abuse (#1 risk factor), critical illness, systemic fungal infection, immunosuppression (from cancer or medications), diabetes, and alcoholism.
Have a high-index of suspicion for endopthalmitis when patients with systemic fungal disease have visual symptoms; endopthalmitis is present in up to 33% of patients with systemic fungal disease.
Symptoms include:
Inspection of both the anterior and posterior chamber is essential to during evaluation; several small yellow-white circular or “fluffy” lesions with surrounding hemorrhage are demonstrated.
Definitive diagnosis made by vitreous biopsy, culture, or PCR; presumptive treatment is acceptable if systemic fungal disease has been demonstrated.
Treatment with Amphotericin B or Voriconazole may be used for broad-spectrum fungal coverage until specific culture and sensitivities return.
Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol. Apr 2008;92(4):466-8.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
Category: Critical Care
Posted: 1/10/2012 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Hypertonic Saline for Intracranial Hypertension
Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care 2011.
Category: Critical Care
Keywords: blunt trauma, vascular inury, anticoagulation, thrombosis, emboli (PubMed Search)
Posted: 1/3/2012 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Carotid or vertebral artery injury following blunt trauma is a rare (%1 of blunt trauma), but a potentially serious injury potentially causing stroke and long-term disability.
Injury leads to an intimal tear becoming a nidus for platelet aggregation; thrombosis and/or distal emboli may subsequently develop.
Mechanisms of injury include:
Symptoms of carotid injury may include contralateral sensorimotor deficits; Symptoms of vertebral injury may include ipsilateral facial pain and numbness, headache, ataxia, or dizziness.
Angiography is the diagnostic “gold standard” but these days a 16-slice CT angiography (or greater) is a reliable screening tool.
Anticoagulation with heparin is the treatment of choice for severe injury, if there are no contraindications (e.g., intracranial bleeding). Anti-platelet drugs may be acceptable in certain cases.
Kim YK, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. Apr 2009;123(6):810-21.
Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. Mar 22 2001;344(12):898-906.
Follow me on Twitter (@criticalcarenow) or Google+ (+haney mallemat)
