Category: Pharmacology & Therapeutics
Keywords: Insulin, Hyperkalemia, Dextrose (PubMed Search)
Posted: 11/6/2017 by Wesley Oliver
(Updated: 2/1/2026)
Click here to contact Wesley Oliver
| Strategies for Hyperkalemia Management | |
| Stabilize cardiac membrane | Calcium gluconate |
| Intracellular movement in skeletal muscles | Albuterol Sodium Bicarbonate Insulin |
| Potassium excretion | Loop Diuretics Kayexalate Patiromer (chronic use only) |
| Potassium removal | Dialysis |
Insulin mechanism of action for hyperkalemia:
· Binds to skeletal muscle receptors
· Increased activity of the sodium-potassium adenosine triphosphatase and glucose transporter GLUT4
· Glycemic response occurs at lower levels of insulin
· Potassium transport activity increases as insulin levels increase
Patients with insulin resistance due to type-2 diabetes do not become resistant to the kalemic effects of insulin.
Hypoglycemia following insulin administration for hyperkalemia:
· Occurs 1-3 hours post dose, even with initial bolus of dextrose
· The amount of glucose is insufficient to replace the glucose utilized in response to the administered dose of insulin
· Insulin’s half-life is increased in ESRD leading to longer duration of action
A systematic review of 11 studies regarding insulin dosing for hyperkalemia:
· 22 patients (18%) experienced hypoglycemia
· Studies that only gave 25 grams (1 amp) of dextrose had the highest incidence of hypoglycemia (30%)
Tips:
· Consider insulin dose reduction in patients with renal failure
· Use an order set to ensure patients receive appropriate POC glucose monitoring to detect delayed onset of hypoglycemia
· Dextrose 50% (25 grams) should be given to all patients with pre-insulin BG <350 mg/dL
Subsequent PRN dextrose 50% (25 grams) should be used to maintain BG >100 mg/dL after insulin administration
References:
1. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney International 2016;89(3):5460554.
2. Harel Z, Kamel KS (2016) Optimal Dose and Method of Administration of Intravenous Insulin in the Management of Emergency Hyperkalemia: A Systematic Review. PLoS ONE 11(5): e0154963. doi:10.1371/journal.pone.0154963
Category: Pharmacology & Therapeutics
Keywords: antipyretic, sepsis, fever (PubMed Search)
Posted: 10/7/2017 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Fever occurs in 40% of patients with sepsis. Historically, there has been conflicting evidence of whether patient outcomes improve with antipyretic therapy.
A recent large meta-analysis assessed the effect of antipyretic therapy on mortality of critically ill septic patients. The analysis included 8 randomized studies (1,531 patients) and 8 observational studies (17,432 patients) that assessed mortality of septic patients with and without antipyretic therapy.
The authors found no difference in mortality at 28 days or during hospital admission. There was also no difference in shock reversal, heart rate, or minute ventilation.
As expected, they found a statistically significant reduction in posttreatment body temperature (-0.38°C, 95% IC -0.63 to -0.13) in patients who received antipyretic therapy. NSAIDs and cooling therapies were more effective than acetaminophen, however no agent or dosing information was provided and only one study included physical cooling therapies.
Bottom Line: Antipyretic therapies do not reduce mortality in patients with sepsis, but they may improve patient comfort by reducing body temperature.
Drewry AM, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med 2017;45:806-813.
Category: Pharmacology & Therapeutics
Keywords: Ureteral stones, Alpha-blockers (PubMed Search)
Posted: 9/2/2017 by Wesley Oliver
(Updated: 2/1/2026)
Click here to contact Wesley Oliver
Alpha-blockers (tamsulosin, alfuzosin, doxazosin, and terazosin) are antagonists of alpha1A-adrenoreceptors, which results in the relaxation of ureteral smooth muscle. Current evidence suggests alpha-blockers may be useful when ureteral stones are 5-10 mm; however, there is no evidence to support the use of alpha-blockers with stones <5 mm. Patients with ureteral stones >10 mm were excluded from studies utilizing these medications.
The size of most ureteral stones will be unknown due to the lack of need for imaging able to measure stone size. Given that the median ureteral stone size is <5 mm, most patients will not benefit from the use of an alpha-blocker.
Also, keep in mind that the data for adverse events with alpha-blockers used for ureteral stones is limited and that these medications have a risk of hypotension.
Ferre RM et al. Tamsulosin for ureteral stones in the emergency department: a randomized, controlled trial. Ann Emerg Med 2009.
77 patients
Ibuprofen + oxycodone + tamsulosin vs. ibuprofen + oxycodone
Stone expulsion at 14 days: Tamsulosin group=77.1% vs. Standard therapy=64.9%
-Difference=12% (95% CI: -8.4-32.8%)
No clinically/statistically significant differences
Pickard R et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet 2015.
1,136 patients
Tamsulosin vs. nifedipine vs. placebo
No further intervention at 4 weeks: Tamsulosin=81% vs. Nifedipine=80% vs. Placebo=80%
No clinically/statistically significant differences
Furyk JS et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med 2016.
403 patients
Tamsulosin vs. placebo
Stone passage at 28 days: Tamsulosin=87% vs. Placebo=81.9%
-Difference=5% (95% CI: -3-13%)
Found difference in subgroup analysis of large stones (5-10 mm)
-Tamsulosin=83.3% vs. Placebo=61%
-Difference=22.4% (95% CI: 3.1-41.6%)
No other clinically/statistically significant differences
Hollingsworth JM et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ 2016.
Meta-analysis of 55 trials
No benefit in patients with smaller stones (<5 mm): RR=1.19 (95% CI: 1.00-1.98)
Benefit in patients with larger stones (5-10 mm): RR=1.57 (95% CI: 1.39-1.61)
1.) Ferre RM, Wasielewski JN, Strout TD, Perron AD. Tamsulosin for ureteral stones in the emergency department: a randomized, controlled trial. Ann Emerg Med 2009;54:432-9.
2.) Furyk JS, Chu K, Banks C, et al. Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med 2016;67:86-95.
3.) Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ 2016;355:i6112.
4.) Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet 2015;386:341-9.
Category: Pharmacology & Therapeutics
Keywords: Levofloxacin, duration, dose, CAP, pneumonia (PubMed Search)
Posted: 7/1/2017 by Jill Logan
(Updated: 2/1/2026)
Click here to contact Jill Logan
When you look up dosing for levofloxacin for community acquired pneumonia (CAP), you will find that both of the following options are approved:
This is based on a multicenter, randomized, double-blind, active treatment trial comparing these two regimens in CAP (mild to severe). This non-inferiority trial shows that the 750 mg dose of levofloxacin for 5 days is "at least as effective and well tolerated" as the 500 mg dose of levofloxacin for 10 days.
So why should you choose the 750 mg daily x 5 day regimen?
As alway with levofloxacin, don't forget to renally dose adjust subsequent doses when writting a script or scheduled inpatient order for patients with reduced creatinine clearance!
Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: A new treatment paradigm. Clin Infect Dis. 2003;37:752-60.
Category: Pharmacology & Therapeutics
Keywords: MSSA, MRSA, bacturia, bacteremia, Staph aureus, Staphlococcus aureus (PubMed Search)
Posted: 6/4/2017 by Jill Logan
(Updated: 2/1/2026)
Click here to contact Jill Logan
Risk factors associated with S. aureus bacturia include:
Al Mohajer M, Darouiche RO. Staphylococcus aureus bacteriura: source, clinical relevance, and management. Curr Infect Dis Rep. 2012;14:601-6.
Category: Pharmacology & Therapeutics
Posted: 4/27/2017 by Tu Carol Nguyen, DO
Click here to contact Tu Carol Nguyen, DO
Haloperidol has a higher D2 receptor antagonist effect than standard antiemetic treatment agents such as metoclopramide. In addition, newer antipsychotic agents such as Olanzapine have a high affinity at multiple antiemetic sites such as the dopamine and serotinergic receptors.
While formal RCT's are still in the works, multiple sources including palliative care, emergency medicine, and pain journals support their use in refractory emesis.
Consider Haloperidol 3-5 mg IV.
Check an EKG for long QTc prior to use. Consider dose reduction of haloperidol in those with hepatic impairment. Also consider dose reduction in patients taking carbamazepine, phenytoin, phenobarbital, rifampicin, or quinidine due to that pesky CYP3A4 inhibition.
Consider Olanzapine 2-5 mg IV.
Several case reports have shown a higher rate of success with olanzapine for refractory emesis. Olanzapine has similar precautions as those to haloperidol (EKG, hepatic impairment), although it's CYP drug interactions are less common. Additionally, use olanzapine cautiously in hyperglycemic patients as there are several case reports of olanzapine prompting episodes of DKA. Consider frequent blood sugar checks or small doses of insulin in hyperglycemic patients.
Take Home Points:
Consider the antipsychotic agents Haloperidol or Olanzapine for patients with refractory emesis, they may be more effective than traditional antiemetics.
Get an EKG prior to administration to check for QTc prolongation. As the classical and atypical antipsychotic agents are sedating, use caution in conjunction with other sedating medications (such as benzodiazepines).
Category: Pharmacology & Therapeutics
Keywords: methadone, linezolid, serotonin syndrome, drug interaction (PubMed Search)
Posted: 4/1/2017 by Michelle Hines, PharmD
(Updated: 4/3/2017)
Click here to contact Michelle Hines, PharmD
Linezolid is a weak, nonselective monoamine oxidase inhibitor (MAOI). A recent FDA Drug Safety Communication released in March 2016 noted reports of serotonin syndrome associated with certain opioids, particularly fentanyl and methadone. Development of serotonin syndrome after concomitant administration of linezolid with other serotonergic agents has been reported. Due to a potential risk of serotonin syndrome, a patient on chronic methadone should not be started on concomitant linezolid unless they will be monitored.
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: NSAID, diazepam, back pain (PubMed Search)
Posted: 3/4/2017 by Michelle Hines, PharmD
(Updated: 2/1/2026)
Click here to contact Michelle Hines, PharmD
The addition of diazepam to naproxen for patients with acute, nontraumatic, nonradicular lower back pain did not improve pain or functional outcomes at 1 week or 3 months after ED discharge compared to placebo.
Study design: single-center, prospective, randomized, double-blind, placebo-controlled trial
Patients:
Treatment groups:
Outcomes:
Results:
Conclusions:
Citation: Friedman BW, Irizarry E, Solorzano C, et al. Diazepam is no better than placebo when added to naproxen for acute low back pain. Ann Emerg Med 2017. PMID 28187918
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: sepsis, antibiotics, vasopressors, shock (PubMed Search)
Posted: 2/4/2017 by Michelle Hines, PharmD
(Updated: 2/1/2026)
Click here to contact Michelle Hines, PharmD
Below is a list of pharmacy-related pearls from the 2016 Surviving Sepsis Guidelines:
Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 3. [PMID 28098591]
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: ketorolac, NSAID, analgesia (PubMed Search)
Posted: 1/7/2017 by Michelle Hines, PharmD
(Updated: 2/1/2026)
Click here to contact Michelle Hines, PharmD
In a study comparing ketorolac IV doses of 10 mg, 15 mg, and 30 mg, no difference in pain score reduction or need for rescue analgesia was observed.
Based upon this study, lower ketorolac doses of 10 mg or 15 mg are equal in analgesic efficacy to a higher dose of 30 mg. A lower dose of 10 mg or 15 mg should be used to avoid adverse effects.
Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med 2016. PMID 27993418
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: esmolol, ventricular fibrillation, cardiac arrest (PubMed Search)
Posted: 11/21/2016 by Michelle Hines, PharmD
(Updated: 12/3/2016)
Click here to contact Michelle Hines, PharmD
Consider esmolol IV 500 mcg/kg loading dose followed by a continuous infusion of 0-100 mcg/kg/min for patients in refractory ventricular fibrillation
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: anticoagulation, warfarin, heparin, bridge, DVT (PubMed Search)
Posted: 11/5/2016 by Michelle Hines, PharmD
Click here to contact Michelle Hines, PharmD
Do you have a patient with renal insufficiency who is in need of an anticoagulation bridge to warfarin? Subcutaneous unfractionated heparin (UFH) as an initial dose of 333 Units/kg subcutaneously followed by a fixed dose of 250 Units/kg (actual body weight) every 12 hours may be an alternative to admission for heparin infusion with monitoring.
Practical Considerations:
Kearon C, Ginsberg JS, Julian JA, et al. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA 2006; 296:935-42. [PMID 16926353]
Morris TA, Jacobson A, Marsh JJ, et al. Pharmacokinetics of UH and LMWH are similar with respect to antithrombin activity. Thromb Res 2005; 115:45-51. [PMID 15567452]
Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST 2012; 141(2)(Suppl):e152S-e184S. [PMID 22315259]
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: QTc prolongation, torsades, antiemetics, antihistamines (PubMed Search)
Posted: 10/1/2016 by Michelle Hines, PharmD
Click here to contact Michelle Hines, PharmD
What they did:
What they found:
Application to clinical practice:
Burdette S, Roppolo LP, Green W, et al. The effect of antiemetics and antihistamines on the QTc interval in emergent dialysis patients with baseline QTc prolongation. J Emerg Med 2016; 51:99-105. (PMID 27614302)
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: FFP,PCC,ICH,warfarin (PubMed Search)
Posted: 9/3/2016 by Michelle Hines, PharmD
Click here to contact Michelle Hines, PharmD
Prothrombin complex concentrate (PCC) and fresh frozen plasma (FFP) are used for INR reversal in patients on vitamin K antagonists (VKA) (e.g., warfarin) with life-threatening bleeding. Guidelines from the Neurocritical Care Society and Society of Critical Care Medicine recommend using PCC over FFP for patients with VKA-associated hemorrhage and an INR >=1.4.
New study-INCH trial:
What they found:
Application to clinical practice:
Frontera JA, Lewin JJ, Rabinstein AA, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage. Neurocrit Care 2016; 24:6-46. (PMID 26714677)
Steiner T, Poli S, Griebe M, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016; 15:566-73. (PMID 27302126)
Follow me on Twitter @mEDPharmD
Category: Pharmacology & Therapeutics
Keywords: amiodarone, procainamide, ventricular tachycardia (PubMed Search)
Posted: 8/6/2016 by Michelle Hines, PharmD
Click here to contact Michelle Hines, PharmD
Amiodarone 150 mg IV over 10 minutes and procainamide IV 20-50 mg/min (up to 17 mg/kg) are two antiarrhythmic medications recommended in the American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care for stable wide QRS complex tachycardia. [1]
What they did:
Multi-center, prospective, randomized, open-label trial comparing the incidence of major cardiac events in the acute treatment of hemodynamically stable patients with wide QRS monomorphic tachycardia (presumed to be VT) using amiodarone 5 mg/kg IV infused over 20 minutes versus procainamide 10 mg/kg IV infused over 20 minutes. [2] The study period was 40 minutes, starting from the beginning of the infusion.
What they found:
Application to clinical practice:
Follow me on Twitter (@mEDPharmD)
Category: Pharmacology & Therapeutics
Keywords: fluoroquinolone, tendon rupture (PubMed Search)
Posted: 7/1/2016 by Michelle Hines, PharmD
(Updated: 7/2/2016)
Click here to contact Michelle Hines, PharmD
Fluoroquinolone antibiotics are used to treat a wide range of infections and as prophylaxis against infection in certain immune compromised patients. In 2008 the FDA issued a boxed warning for tendonitis and tendon rupture for the fluoroquinolone antibiotic class, and in May 2016 a statement recommending the use of alternate therapies for uncomplicated UTIs and upper respiratory infections was issued. The mechanism by which fluoroquinolones causes tendon injury has not been elucidated, but may be related to oxidative stress caused by the overproduction of reactive oxygen species in tenocytes.
Adverse event reporting to the FDA is performed voluntarily by healthcare professionals and consumers through MedWatch. An analysis of tendon rupture events associated with fluoroquinolone use reported to the FDA’s Adverse Event Reporting System (FAERS) database was recently published.
What they found:
Application to clinical practice:
Arabyat RM, et al. Fluoroquinolone-associated tendon-rupture: a summary of reports in the Food and Drug Administration’s adverse event reporting system. Expert Opin Drug Saf 2015; 14:1653-60. (PMID 26393387)
FDA Drug Safety Communication from 5/12/2016: http://www.fda.gov/Drugs/DrugSafety/ucm500143.htm
Follow me on Twitter (@mEDPharmD)
Category: Pharmacology & Therapeutics
Keywords: clindamycin, trimethoprim-sulfamethoxazole, wound infection, TMP-SMX (PubMed Search)
Posted: 6/2/2016 by Bryan Hayes, PharmD
(Updated: 6/4/2016)
Click here to contact Bryan Hayes, PharmD
In settings where community-acquired MRSA is prevalent, which antibiotic is best for uncomplicated wound infections?
New Study
What They Found
Application to Clinical Practice
Talan DA, et al. A randomized trial of clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated wound infection. Clin Infect Dis 2016;62(12):1505-13. [PMID 27025829]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: ketamine, shock index, hemodynamic, prehospital, RSI (PubMed Search)
Posted: 5/3/2016 by Bryan Hayes, PharmD
(Updated: 5/7/2016)
Click here to contact Bryan Hayes, PharmD
Ketamine is often thought to be the induction agent least associated with hypotension in the peri-intubation period. However, reports of hypotension following ketamine do exist, including 2 cases of cardiac arrest. [1] There are limited objective means to predict which patients may have an adverse hemodynamic response.
New Study
A new prospective observational study followed 112 patients in the prehospital setting who received ketamine for rapid sequence intubation. 81 had a low shock index [< 0.9], 31 had a high shock index. [2]
Shock index = HR / SBP
What They Found
Patients with a high shock index were more likely to experience hypotension (SBP < 90 mm Hg) in the peri-intubation period compared to those with a low shock index (26% vs 2%).
Application to Clinical Practice
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: vancomycin, loading dose, nephrotoxicity (PubMed Search)
Posted: 3/24/2016 by Bryan Hayes, PharmD
(Updated: 4/2/2016)
Click here to contact Bryan Hayes, PharmD
Guidelines recommend loading doses of vancomycin (15-20 mg/kg, up to 30 mg/kg in critically ill patients), but the risk of nephrotoxicity is unknown. A new retrospective cohort study aimed to compare nephrotoxicity in ED sepsis patients who received vancomycin at high doses (>20 mg/kg) versus lower doses (20 mg/kg).
What They Found
1,330 patients had three SCr values assessed for the primary outcome
High-dose initial vancomycin was actually associated with a lower rate of nephrotoxicity (5.8% vs 11.1%)
After adjusting for age, gender, and initial SCr, the risk of high dose vancomycin compared to low dose was decreased for the development of nephrotoxicity (RR=0.60; 95% CI: 0.44, 0.82)
Application to Clinical Practice
It appears initial loading doses of vancomcyin > 20 mg/kg do not cause increased risk of nephrotoxicity.
Rosini JM, et al. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad Emerg Med. 2016 Feb 6. Epub ahead of print. [PMID 26850378]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
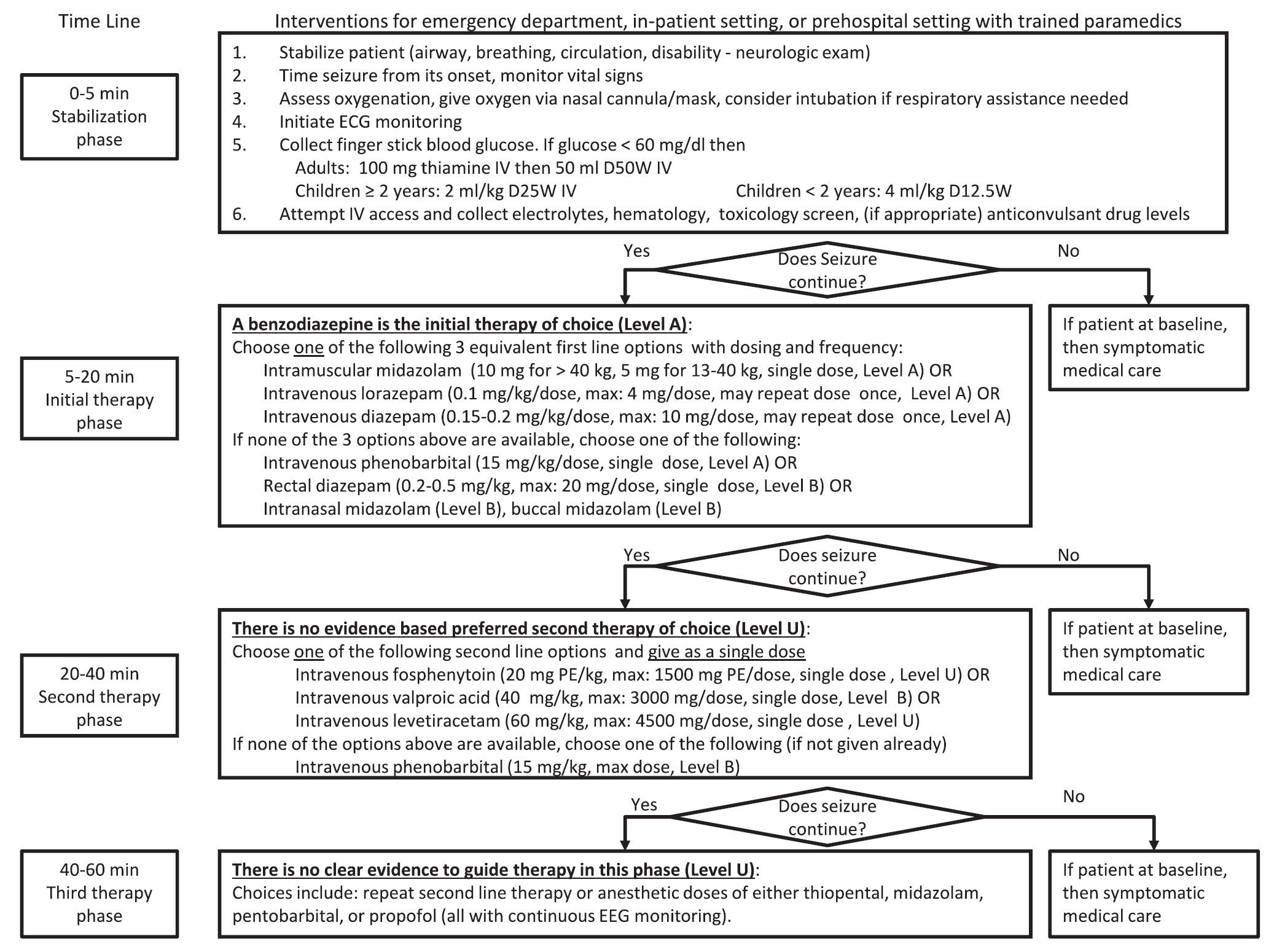
Keywords: status epilepticus (PubMed Search)
Posted: 3/3/2016 by Bryan Hayes, PharmD
(Updated: 3/5/2016)
Click here to contact Bryan Hayes, PharmD
A new guideline for convulsive status epilepticus in adults AND children was recently published. [1] An insightful commentary was published alongside it (both are open access). [2] The proposed algorithm is below. Here are a few additional points to note:
Follow me on Twitter (@PharmERToxGuy)
