Category: Pharmacology & Therapeutics
Keywords: complicated UTI, urinary tract infection, UTI, pyelonephritis, cystitis (PubMed Search)
Posted: 1/6/2026 by Alicia Pycraft
(Updated: 1/8/2026)
Click here to contact Alicia Pycraft
Previous guidelines for the treatment of urinary tract infections (UTI) were published in 2010 and focused on treatment of uncomplicated cystitis and pyelonephritis in women. Due to lack of published evidence at the time, these guidelines notably omitted discussion of complicated UTI (cUTI) and UTI in men. In July 2025, the Infectious Diseases Society of America (IDSA) released new, long-awaited guidelines for the treatment of cUTI. Below are key guideline updates to consider in the treatment of patients with cUTI presenting to the emergency department:
Bottom line: UTIs in males are no longer considered inherently complicated, treatment should be selected among preferred antimicrobials using a 4-step approach, and shorter (5-7 day) antibiotic courses may be considered for some patients with cUTI. As always, consult with your local antibiogram or pharmacist for guidance!
Category: Pharmacology & Therapeutics
Posted: 12/9/2025 by Ashley Martinelli
(Updated: 12/11/2025)
Click here to contact Ashley Martinelli
Sympathetic crashing acute pulmonary edema (SCAPE) is an acute, aggressive pulmonary edema that occurs in patients with hypertensive emergencies. Nitroglycerin (NTG) is often utilized in combination with non-invasive positive pressure ventilation to prevent decompensation; however, data is lacking regarding the optimal dosing strategy.
Study design: retrospective, single-center, cohort study at an academic medical center
Inclusion: adult patients with a primary or secondary diagnosis of pulmonary edema, acute heart failure exacerbation, hypertensive emergency, or hypertensive crisis and were initiated on NTG in the ED.
Exclusion: hypertensive emergency with different BP goals (dissection, eclampsia, ICH)
Study groups: based on initial NTG dose (<100 mcg/min = low dose, ? 100 mcg/min = high dose)
Primary outcome: time from NTG initiation to oxygen weaning (removal of necessary oxygen back to baseline or home oxygen
Baseline: 61 years old, 50% male, 97% with history of hypertension, 84% history of heart failure, and 36% with ESRD. A higher percentage of patients in the high dose group has CPAP/BIPAP (49% vs 27% p<0.001)
Results: High dose NTG group had a shorter time from NTG to oxygen wean of 2.67h compared to 3.28 hours in the low group. The high dose group also was more likely to achieve goal SBP reduction of 25% within the hour (55% v 34%, p<0.001) had a shorter duration of NTG infusion overall 4.9h vs 6.9h, p0.033) and had decreased ICU LOS by 0.5 days. There were more cases of hypotension in the high dose group which was primarily driven by acute drops in SBP >30%.
Bottom Line: Consider using NTG 100 mcg/min initially to manage patients with SCAPE in the ED.
Henry K, Pelsue B, Hartman H, Gulbis B. Low versus high dosing strategies of intravenous nitroglycerin for the management of sympathetic crashing acute pulmonary edema. Am J Emerg Med. 2025; 98:41-45.
Category: Pharmacology & Therapeutics
Keywords: andexanet alfa, 4F-PCC, Kcentra, ICH, thrombosis (PubMed Search)
Posted: 11/13/2025 by Wesley Oliver
(Updated: 2/1/2026)
Click here to contact Wesley Oliver
This pearl was adapted from a literature update presented by Castin Schulz, PharmD on November 13, 2025.
A 2025 study in the American Journal of Emergency Medicine provides new real-world data on the two most common reversal agents for factor Xa (fXa) inhibitor-related intracranial hemorrhage (ICH).
This national retrospective cohort study evaluated 350 Veterans who received either andexanet alfa (AA) or 4-factor prothrombin complex concentrate (4F-PCC) for fXa inhibitor-related ICH.
Key Findings (Propensity-Matched Analysis)
Clinical Takeaway
In this study of Veterans with fXa inhibitor-related ICH, andexanet alfa did not improve 90-day mortality compared to 4F-PCC. However, its use was associated with a significantly increased risk of 30-day thrombotic events, particularly ischemic stroke.
This study adds to a growing body of literature questioning the safety profile of AA. The authors conclude that the selection of AA should be carefully weighed against the patient's underlying risk of thrombotic events.
Rech MA, Budde E, Evans CT, et al. Andexanet alfa increases 30-day thrombotic events relative to four-factor prothrombin complex concentrate for factor Xa inhibitors-related intracerebral hemorrhage in veterans. Am J Emerg Med. 2025;97:97-102. doi:10.1016/j.ajem.2025.07.037
Category: Pharmacology & Therapeutics
Keywords: rapid sequence intubation, rocuronium, paralytic, awareness (PubMed Search)
Posted: 10/9/2025 by Alicia Pycraft
Click here to contact Alicia Pycraft
It is estimated that between 2.6% and 3.4% of patients undergoing rapid sequence intubation (RSI) experience awareness with paralysis, with the highest risk observed in patients receiving rocuronium. Several studies have now demonstrated prolonged time to sedation following RSI with long-acting paralytics, including a 2024 single-centered retrospective chart review by Dukes et al., which found that fewer than half of patients in the ICU receiving rocuronium for RSI were administered sedation within 15 minutes of intubation. The following study by Cappuccilli et al. sought to compare differences in sedation practices between the ED and ICUs at the same institution, hypothesizing that patients undergoing RSI in the ED would experience similar delays in sedation to those in the ICU.
Category: Pharmacology & Therapeutics
Keywords: acute ischemic stroke, tenecteplase, thrombolytic, endovascular treatment, large?vessel occlusion (PubMed Search)
Posted: 9/11/2025 by Matthew Poremba
Click here to contact Matthew Poremba
Background:
Several trials have explored the use of IV thrombolysis before endovascular thrombectomy (EVT) in ischemic stroke patients, and a pooled analysis from these trials showed no significant difference in efficacy between intravenous thrombolysis plus EVT compared to EVT alone. However, only 2.2% of patients in the trials included in this pooled analysis received tenecteplase (TNK), with the vast majority of patients receiving alteplase. (1) While a 2018 trial showed improved early reperfusion and 90-day outcomes with TNK compared to alteplase before EVT, a recent target trial emulation analysis indicated no added benefit with TNK plus EVT over EVT alone. (2-3) Lack of comparison between TNK plus EVT versus EVT alone and small sample sizes of prior trials led to the design of the BRIDGE-TNK trial, which directly compared TNK plus EVT versus EVT alone in acute ischemic stroke. (4)
Study design:
This multi-center, randomized, open-label trial conducted at 39 hospitals in China included patients with large-vessel occlusion (LVO) of the internal carotid, middle cerebral or basilar artery on CTA or MRA imaging who presented within 4.5 hours of their last known well time, and were eligible to undergo intravenous thrombolysis and EVT.
Exclusion criteria were intracranial hemorrhage on CT or MRI imaging, rapidly improving symptoms at the discretion of the investigator, pre-stroke modified Rankin scale (mRS) of > 4, contraindication to imaging with contrast agents, patients who needed interhospital transfer before thrombectomy, any terminal illness such that the patient would not be expected to survive more than 1 year, any condition that could impost hazards to the patient if study therapy is initiated in the judgement of the investigator, hypodensity in >1/3 of middle cerebral artery or basilar artery territory on non-contrast CT, and pregnant women.
The primary outcome was functional independence at 90 days, defined as an mRS score of 0 to 2.
Patient Population:
Baseline characteristics were well matched between treatment arms:
Results:
Primary outcome:
Secondary outcomes:
Study Limitations:
Key Takeaways:
While rates of symptomatic intracranial hemorrhage and mortality were higher in the TNK + EVT group, neither of these outcomes met statistical significance and bridging with TNK prior to EVT led to increased rates of functional independence at 90 days compared to EVT alone. The findings of this study reinforce current guideline recommendations for not skipping intravenous thrombolysis prior to thrombectomy for LVO stroke.
1. Majoie CB, Cavalcante F, Gralla J, et al. Value of intravenous thrombolysis in endovascular treatment for large-vessel anterior circulation stroke: individual participant data meta-analysis of six randomised trials. Lancet 2023;402:965-74
2. Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 2018;378:1573-82
3. Altersberger VL, Kaesmacher J, Churilov L, et al. Bridging thrombolysis with tenecteplase versus endovascular thrombectomy alone for large-vessel anterior circulation stroke: a target trial emulation analysis. J Neurol Neurosurg Psychiatry. 2025;96(8):775-783. Published 2025 Jul 16. Doi:10.1136/jnnp-2024-335325
4. Qiu Z, Li F, Sang H, et al. Intravenous Tenecteplase before Thrombectomy in Stroke. N Engl J Med. 2025;393(2):139-150. doi:10.1056/NEJMoa2503867d
Category: Pharmacology & Therapeutics
Keywords: steroids, asthma, copd (PubMed Search)
Posted: 8/7/2025 by Ashley Martinelli
(Updated: 8/14/2025)
Click here to contact Ashley Martinelli
There are various reasons to give corticosteroids in the emergency department. Many decisions regarding IV vs PO, and the numerous available products can lead to excessive dosing (such as 125mg methylprednisolone). Below is a reference for the most common indications as well as conversion recommendations for each product
Guideline Recommended Dosing for Common ED Indications:
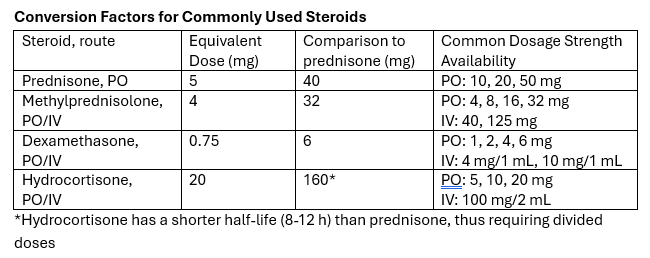
Take-away: Methylprednisolone 125mg is frequently requested but provides a dose equivalent to prednisone 150mg. Consider guideline directed dosing and conversion of products to prevent excessive initial steroid dosing.
Category: Pharmacology & Therapeutics
Keywords: sepsis, beta-lactam, vancomycin, antibiotic (PubMed Search)
Posted: 6/13/2025 by Alicia Pycraft
Click here to contact Alicia Pycraft
Background:
Early antibiotic administration is consistently linked to improved mortality outcomes in patients with sepsis. As a result, time-to-antibiotic delivery is a critical metric in hospital sepsis quality improvement initiatives. Empiric treatment often consists of a broad-spectrum beta-lactam to cover both gram-positive and gram-negative organisms, alongside vancomycin to ensure coverage of methicillin-resistant Staphylococcus aureus (MRSA). When multiple agents are indicated, they may be given simultaneously; however, factors such as limited intravenous (IV) access or drug incompatibilities can necessitate sequential administration. Administration of vancomycin first may delay the administration of a beta-lactam agent by at least 60-120 minutes due to its prolonged infusion time. This raises an important clinical question: Does the order in which antibiotics are administered influence outcomes in sepsis?
A 2022 retrospective study by Amoah et al. found that, among patients with confirmed bloodstream infections, a beta-lactam-first regimen was associated with a 52% reduction in the odds of short-term mortality compared to a vancomycin-first regimen. However, the generalizability of these findings to the broader population of patients with suspected sepsis, of whom only 15-20% ultimately have positive blood cultures, remains uncertain.
What's new?
A recent retrospective, multi-center, cohort study by Kondo et al. evaluated the impact of a beta-lactam-first antibiotic strategy compared to a vancomycin-first strategy on in-hospital mortality in patients with suspected sepsis. Of the 25,391 patients with sepsis who were screened, 21,449 (84.4%) received a beta-lactam first and 3,942 (15.6%) received vancomycin first. Patients who received vancomycin first had lower comorbidity burden, lower illness severity, more skin/musculoskeletal infections, and received beta lactams a median of 3.5 hours later relative to ED arrival compared to those who received a beta-lactam first. Although the overall rate of documented bloodstream infections was similar between groups, MRSA-positive cultures were more common in the vancomycin-first group, both in clinical cultures (4.5% vs. 3.2%) and in blood cultures (1.8 vs. 1.2%).
Beta-lactam administration prior to vancomycin was associated with an 11% reduction in the odds of in-hospital mortality (aOR: 0.89; 95% CI: 0.8-0.99; p=0.046). When the time-to-first antibiotic covariate was replaced with time-to-first beta-lactam, this association was no longer significant (aOR 0.93, 95% CI: 0.82-1.05, p=0.25), suggesting a possible link between time-to-first beta-lactam antibiotic and mortality. There was a trend toward lower in-hospital morality for the beta-lactam first regimen in several subgroups examined, including patients with positive blood cultures or positive MRSA cultures, and patients who received anti-pseudomonal beta-lactams; however, none reached statistical significance.
Bottom line:
Given the observed mortality benefit and absence of harm associated with a beta-lactam-first approach, even among patients with positive MRSA cultures, the findings of this study support the prioritization of beta-lactam therapy in patients with sepsis.
Category: Pharmacology & Therapeutics
Keywords: urinary tract infection, pyelonephritis, cephalosporins, fluoroquinolones, antimicrobial resistance (PubMed Search)
Posted: 5/8/2025 by Matthew Poremba
Click here to contact Matthew Poremba
Background:
The 2010 Infectious Diseases Society of America (IDSA) cystitis and pyelonephritis guidelines recommend fluoroquinolones (FQs) as first line agents for pyelonephritis treatment and also support trimethoprim-sulfamethoxazole (TMP-SMX) usage if the urinary pathogen is known to be susceptible. However, alternative regimens need to be evaluated as FQs are increasingly associated with serious adverse events, and E coli resistance rates to both FQs and TMP-SMX are rising nationally. The Cephalosporins for Outpatient Pyelonephritis in the Emergency Department (COPY-ED) study aimed to evaluate the effectiveness of oral cephalosporins in acute pyelonephritis treatment when compared to IDSA guideline-endorsed first line treatments.
Study design:
This multicenter, retrospective observational cohort study screened patients with a primary diagnosis of uncomplicated or complicated pyelonephritis using ICD-10 codes. They included all patients >18 years of age who reported symptoms of a UTI and were discharged home on oral antimicrobial therapy. Exclusion criteria included pregnancy, acute or chronic prostatitis, orchitis, epididymitis, or urinary tract surgery within 7 days prior to ED visit or surgery planned during the study period.
The primary outcome was rate of outpatient treatment failure within 14 days of discharge from the emergency department with cephalosporins compared to FQs and TMP-SMX.
Patient Population:
Results:
Rates of treatment failure at 14 days were not statistically significant between groups, with a rate of 17.2% in the cephalosporin group and a rate of 22.5% in the FQ + TMP/SMX group. After adjusting for gender, complicated infections, previous use of intravenous or oral antibiotics, and urinary tract abnormality, the odds of treatment failure at 14 days were still not significantly different in patients who received fluoroquinolone or TMP/SMX (adjusted OR 1.275 [95% CI 0.86 to 1.9]) compared to cephalosporins.
Secondary outcomes included rates of treatment failure with first generation cephalosporins (cephalexin, cefadroxil, cefuroxime) and third generation cephalosporins (cefpodoxime, cefuroxime), rates of appropriate therapy selected based on urine culture susceptibilities, and rates of treatment failure compared to duration of treatment prescribed. None of these outcomes found statistically significant differences between groups.
Study Limitations:
Key Takeaways:
Category: Pharmacology & Therapeutics
Keywords: Albuterol, Lactate (PubMed Search)
Posted: 4/10/2025 by Wesley Oliver
(Updated: 2/1/2026)
Click here to contact Wesley Oliver
Albuterol, a common bronchodilator used in the treatment of asthma and chronic obstructive pulmonary disease (COPD), can cause a surprising increase of lactate levels. The increase in lactate is usually mild to moderate (typically < 4 mmol/L) and transient. It does not necessarily indicate underlying sepsis, tissue hypoxia, or severe metabolic acidosis.
Mechanism:
Albuterol can cause a transient increase in lactate levels due to its beta-2 agonist effects, which promote glycogenolysis and increase anaerobic metabolism. This can result in elevated lactic acid production, even in the absence of tissue hypoxia or shock.
Timing:
This effect is typically seen within 30 minutes of albuterol administration and can persist for 1-2 hours after discontinuing treatment.
Monitoring:
If lactate levels are elevated in a patient receiving albuterol, consider the possibility of a pharmacologic cause rather than immediately assuming a more serious etiology like shock or severe metabolic disturbance.
Differentiating Causes of Elevated Lactate:
In a critically ill patient, elevated lactate can indicate hypoperfusion (e.g., septic shock, cardiogenic shock, or hypovolemic shock). However, when elevated lactate is associated with albuterol administration, the rise in lactate is often lower and resolves without intervention.
Management:
If albuterol-induced lactate elevation is suspected, continue with supportive care and monitor lactate trends. No specific treatment is necessary for the elevated lactate unless there are other concerning clinical findings that suggest a different underlying cause.
Conclusion:
In emergency settings, it's important to recognize that albuterol can cause a transient increase in lactate levels. Understanding this phenomenon can help avoid misdiagnosis and prevent unnecessary interventions in patients receiving albuterol therapy. Always correlate lactate levels with the broader clinical picture to guide management decisions.
Hockstein M, Diercks D. Significant Lactic Acidosis from Albuterol. Clin Pract Cases Emerg Med. 2018 Mar 14;2(2):128-131. doi: 10.5811/cpcem.2018.1.36024. PMID: 29849230.
Lewis LM, Ferguson I, House SL, Aubuchon K, Schneider J, Johnson K, Matsuda K. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014 Jan;145(1):53-59. doi: 10.1378/chest.13-0930. PMID: 23949578.
Liedtke AG, Lava SAG, Milani GP, Agostoni C, Gilardi V, Bianchetti MG, Treglia G, Faré PB. Selective ß2-Adrenoceptor Agonists and Relevant Hyperlactatemia: Systematic Review and Meta-Analysis. J Clin Med. 2019 Dec 27;9(1):71. doi: 10.3390/jcm9010071. PMID: 31892109.
Maeda T, Paralkar J, Kuno T, Patrawalla P. Inhaled Albuterol Use and Impaired Lactate Clearance in Patients With Sepsis: A Retrospective Cohort Study. J Intensive Care Med. 2021 Mar;36(3):284-289. doi: 10.1177/0885066619901095. Epub 2020 Jan 22. PMID: 31964210.
Zitek T, Cleveland N, Rahbar A, Parker J, Lim C, Elsbecker S, Forred W, Slattery DE. Effect of Nebulized Albuterol on Serum Lactate and Potassium in Healthy Subjects. Acad Emerg Med. 2016 Jun;23(6):718-21. doi: 10.1111/acem.12937. Epub 2016 May 11. PMID: 26857949.
Category: Pharmacology & Therapeutics
Keywords: tenecteplase, alteplase, stroke (PubMed Search)
Posted: 3/10/2025 by Ashley Martinelli
(Updated: 3/13/2025)
Click here to contact Ashley Martinelli
On March 3, 2025, the FDA approved tenecteplase to treat acute ischemic stroke. Historically, only alteplase was FDA-approved, but the stroke guidelines suggest tenecteplase as a reasonable alternative and many centers have made the change to use tenecteplase.
The EXTEND-IA TNK trial showed benefit of tenecteplase over alteplase in patients who were candidates for mechanical thrombectomy. The newer AcT trial found that tenecteplase was non-inferior to alteplase for patients eligible for thrombolysis, regardless of thrombectomy candidacy. There was no difference in safety outcomes, specifically ICH or angioedema in either trial.
Tenecteplase will soon be available in a new 25 mg vial with stroke-specific packaging (potentially as early as June 2025). Currently, there is only a 50 mg vial that is used for STEMI and PE which has higher maximum dosing compared to stroke.
The dosing is now recommended in weight-based groupings based on the supplemental appendix from the AcT trial. This is likely a change in practice for most centers that previously implemented tenecteplase for stroke before the FDA approval. Consult with your stroke and pharmacy team to discuss potential protocol changes at your institution.

Campbell BCV, et al. NEJM 2018;378:1573-1582.
Menon BK, et al. Lancet 2022;400:161-69.
TNKase [package insert]. South San Francisco, CA. Genetech, Inc. 2025.
Genetech Press Release: https://www.gene.com/media/press-releases/15053/2025-03-03/fda-approves-genentechs-tnkase-in-acute-#:~:text=South%20San%20Francisco%2C%20CA%20%2D%2D,stroke%20(AIS)%20in%20adults.
Category: Pharmacology & Therapeutics
Keywords: penicillin, beta-lactam, antibiotic stewardship, allergy, hypersensitivity (PubMed Search)
Posted: 2/13/2025 by Matthew Poremba
Click here to contact Matthew Poremba
Background:
Approximately 10% of patients presenting to the emergency department (ED) report penicillin allergies, which may lead to use of second- or third-line agents. Alternative therapies (such as aztreonam, clindamycin and fluroquinolones) carry an increased risk of mortality and complications such as Clostridioides difficile infection. Considering that less than 10% of penicillin allergies may be confirmed by formal testing results, the PEN-FAST clinical decision tool was created to identify patients with low risk of true penicillin allergy who do not require formal skin testing for rechallenging with a beta-lactam:

Though PEN-FAST has only been validated in the clinic and inpatient settings, a study from Tran et al. published this January sought to determine the safety and efficacy of utilizing this tool to assess penicillin allergies and re-challenge patients in the ED.
Study design:
This was a single-center, prospective, observational cohort study. Emergency medicine (EM) pharmacists screened patients in the ED with:
Screened patients were excluded from the study if orders were placed by a non-EM physician, if they previously tolerated a penicillin/cephalosporin within the healthcare system of the study site, if they were unable to participate in bedside interview, if the antibiotics selected were appropriate despite the penicillin allergy or if there were time constraints that would delay care if the PEN-FAST assessment needed to be completed.
Study Intervention:
EM pharmacists completed the PEN-FAST assessment for all included patients. They recommended rechallenging with an appropriate beta-lactam for patients with a score of 0-2, recommended to consider rechallenging for patients scoring 3, and did not recommend rechallenging for scores of 4-5 or if it was confirmed patients previously experienced anaphylaxis, angioedema or severe cutaneous reactions with a beta-lactam. Orders for any change in therapy were only placed with discussion and agreement from EM physicians. Rechallenged patients were assessed at bedside for any immune-mediated reactions 45 to 75 minutes after initiation of antibiotics. The primary outcome was the percent of patients with a PEN-FAST score of 0-2 who tolerated a beta-lactam after being rechallenged.
Patient Characteristics:
After screening, one hundred patients were included in this study.
Results:
Primary Outcome:
Secondary Outcomes:
Key Takeaways:
Category: Pharmacology & Therapeutics
Keywords: tpa, frostbite, iloprost, therapy (PubMed Search)
Posted: 1/13/2025 by Robert Flint, MD
(Updated: 2/1/2026)
Click here to contact Robert Flint, MD
This meta analysis of studies looking at thrombolytics and prostaglandins in treating significant frost bite offers some insight into the possibilities these therapeutics offer. Unfortunately, the studies available are not high quality and most are case reports.
“Our results suggest that thrombolysis or intravenous iloprost is effective when administered promptly to treat severe frostbite. For grade 3–4 frostbite the Wilderness Medical Society frostbite guidelines recommend the use of intravenous iloprost within 48 h of injury, and thrombolysis within 24 h of injury. The Helsinki protocol recommends the use of tPA for patients with grade 3–4 frostbite presenting within 48 h of injury with angiographic evidence of thrombosis."
“Iloprost is a synthetic prostaglandin I2 that has been used to treat frostbite . Like other prostacyclins, it inhibits platelet aggregation and promotes vasodilation. Iloprost may stimulate the release of endogenous tissue plasminogen activator or counteract its inhibitory effects [35]. Iloprost reduces vasoconstriction induced by thromboxane A2 , and may reduce oxidative stress from free radicals, moderating reperfusion injury [37, 38]. The effect on platelet aggregation may be reversed within two hours), but prostacyclin effects may disrupt the vicious cycle of activated platelets and leukocytes that damages endothelium .”
More research in this area is needed. Transfer to a center with these capabilities seems worth a discussion in the case of severe frostbite.
Regli, I.B., Oberhammer, R., Zafren, K. et al. Frostbite treatment: a systematic review with meta-analyses. Scand J Trauma Resusc Emerg Med 31, 96 (2023). https://doi.org/10.1186/s13049-023-01160-3
Category: Pharmacology & Therapeutics
Keywords: olanzapine, benzodiazepine, drug interaction (PubMed Search)
Posted: 1/10/2025 by Alicia Pycraft
Click here to contact Alicia Pycraft
Background
Treatment of acute agitation often involves combining antipsychotics and benzodiazepines. Injectable olanzapine, a second-generation antipsychotic, uniquely carries a warning against concomitant use with parenteral benzodiazepines. The olanzapine prescribing information states that “concomitant administration of intramuscular (IM) olanzapine and parenteral benzodiazepines is not recommended due to the potential for excessive sedation and cardiorespiratory compromise”. The European Medicines Agency (similar to the United States FDA) cautions against use of the two within 60 minutes of each other using similar language.
The above warnings were based on a 2010 publication of 160 adverse event reports from a post-marketing database maintained by the drug manufacturer, and have resulted in many institutions prohibiting co-administration of IM olanzapine and parenteral benzodiazepines. The publication cited 29 fatal adverse events involving injectable olanzapine, concluding that caution should be exercised when using IM olanzapine and parenteral benzodiazepines simultaneously. However, 25 of the 29 patients received other sedating medications in addition to olanzapine and benzodiazepines, and the majority of fatalities were >12 hours after the last dose of olanzapine. Following this publication, a 2013 randomized controlled trial by Chan et al. found no difference in adverse event rates between patients receiving IV midazolam alone and patients receiving IV midazolam plus IV olanzapine for acute agitation.
This December 2024 study by Cole et al. aimed to re-evaluate the risks of cardiorespiratory compromise with concomitant injectable olanzapine and injectable benzodiazepine administration.
Study design
This was a single-center retrospective cohort study of 693 patients who received 2 parenteral doses of eligible sedating medications within 60 minutes of each other. A total of 549 patients received 2 doses of olanzapine, and 144 received olanzapine and a benzodiazepine (midazolam, lorazepam, or diazepam). To avoid cohorts with a higher baseline risk of sedation, patients who received other sedating medications and patients who received more than 2 doses of olanzapine or 1 dose of a benzodiazepine were excluded.
Patient Population
Results

*One death during hospitalization was due to missed occlusion myocardial infarction
Study Critique:
Key Takeaways
Category: Pharmacology & Therapeutics
Keywords: Antibody-drug conjugates, toxicities, adverse effects (PubMed Search)
Posted: 12/11/2024 by Wesley Oliver
Click here to contact Wesley Oliver
A recent review article highlighted the adverse effects that emergency physicians should know of with the novel antineoplastic agents. The adverse effects and the associated agents are briefly summarized from the article in the table below. A link to the full article is below.
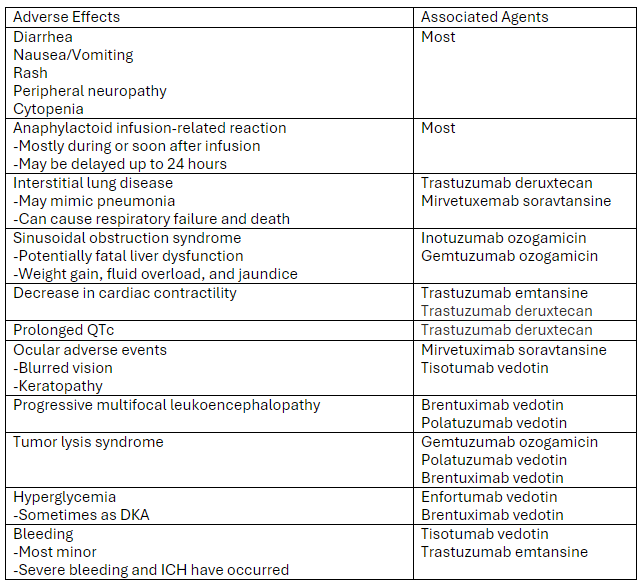
Link to article: Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know - Annals of Emergency Medicine
Markides DM, Hita AG, Merlin J, Reyes-Gibby C, Yeung SJ. Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know. Ann Emerg Med. 2024 Dec 3:S0196-0644(24)01142-9. doi: 10.1016/j.annemergmed.2024.10.015. Epub ahead of print. PMID: 39641680.
Category: Pharmacology & Therapeutics
Keywords: Epinephrine, Allergic Reactions, Anaphylaxis (PubMed Search)
Posted: 10/10/2024 by Matthew Poremba
(Updated: 2/1/2026)
Click here to contact Matthew Poremba
Background:
Epinephrine administration is a critical component of treating severe allergic reactions, and delayed administration is associated with increased morbidity and mortality. Epinephrine auto-injectors are the current standard of care and allow for rapid administration in all care settings, but compliance issues can limit their use. The most common reason patient’s site for failure to administer or delayed administration of auto-injectors is needle phobia (particularly with pediatric patients). This has led to interest in developing needle-free epinephrine delivery devices that are easy to administer.
New Drug Approval:
This August, the FDA approved an epinephrine nasal spray (brand name: Neffy) for use as emergency treatment for Type 1 allergic reactions, including life-threatening anaphylaxis. The approval was based on four studies, including 175 total patients, comparing epinephrine 2 mg nasal spray with an epinephrine 0.3 mg intramuscular injection in healthy adults and children. These studies showed similar blood concentrations of epinephrine between treatment arms through 60 minutes after administration. In addition, both treatment arms showed similar elevations in heart rate and systolic blood pressure.
Bottom Line:
Epinephrine nasal spray is a newly approved option for the treatment of severe allergic reactions and anaphylaxis. While this approval was based on studies in healthy adults and children who did not currently have anaphylaxis, this medication may be worth considering for patients who have issues or concerns about using an injectable device to administer epinephrine.
Category: Pharmacology & Therapeutics
Keywords: hyperkalemia, calcium, cardiac conduction, resting membrane potential (PubMed Search)
Posted: 9/11/2024 by Alicia Pycraft
(Updated: 9/12/2024)
Click here to contact Alicia Pycraft
The benefits of calcium treatment for hyperkalemia have historically been attributed to “membrane stabilization,” as it has been hypothesized to restore cardiac resting membrane potential. However, the true mechanism by which calcium improves cardiac function in this setting remains unclear. This has led to inconsistencies in the clinical threshold for treating hyperkalemia with calcium.
Piktel et al. recently conducted an experimental study investigating the adverse electrophysiologic effects of hyperkalemia and therapeutic effects of calcium treatment in isolated canine myocytes using ex vivo tissue and in vivo cellular techniques.
Key study findings:
Effects of hyperkalemia:
Effects of calcium treatment in the setting of hyperkalemia:
Limitation:
Bottom line: Findings of this study suggest that calcium's beneficial effects in hyperkalemia are not attributed to “membrane stabilization,” but rather to restoration of conduction velocity through L-type calcium channels and subsequent narrowing of the QRS complex. This supports calcium treatment in hyperkalemia when the ECG shows conduction slowing and QRS widening.
Piktel JS, Wan X, Kouk S, Laurita KR, wilson LD. Beneficial effect of calcium treatment for hyperkalemia is not due to “membrane stabilization” Crit Care Med. 2024; 52(00): 1-10.
Category: Pharmacology & Therapeutics
Keywords: Hyponatremia, Correction, 3% Sodium Chloride, Hypertonic Saline (PubMed Search)
Posted: 7/11/2024 by Wesley Oliver
Click here to contact Wesley Oliver
At our institution we have developed a guideline for the use of hypertonic saline in hyponatremia.
Administration of 3% sodium chloride for acute or symptomatic hyponatremia
Acute hyponatremia with severe symptoms
Acute hyponatremia with moderate symptoms
Hyponatremia Fluid Rate Calculations (**Be Careful with Online Calculators**)
FYI: 3% Sodium Chloride (1.95 mL/mEq; 513 mEq/1 L); 0.9% Sodium Chloride (6.5 mL/mEq; 154 mEq/1 L)
Equations for Calculations
***See Visual Diagnosis for an Example with Calculations***
Example:
70 kg male patient with a current sodium of 115 mEq/L (not hyperglycemic)
3% Sodium Chloride
0.9% Sodium Chloride
**Popular Online Calculator Using Same Example**
3% sodium chloride: 54 mL/hr
0.9% sodium chloride: 551 mL/hr
Be aware that the default setting of the calculator is to correct by 12 mEq/L over 24 hours leading to larger rates of infusion.
Adult Hypertonic Aline for Use in Hyponatremia, Medication Use Guideline. University of Maryland Medical System. Accessed July 2024.
Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines.
JASN. 2017; 28(5):1340-1349.
Jones GN, Bode L, Riha H et al. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care. 2017; 26(1): 37-42.
Sodium chloride preparations. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com. Accessed June 2018.
Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatremia. Intensive Care Med. 2014; 40:320-331.
Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, Evaluation and Treatment of Hyponatremia: Expert Panel Recommendations. Amer J Med. 2013; 126:S1-S42.
Category: Pharmacology & Therapeutics
Keywords: alcohol use disorder, phenobarbital, naloxone, treatment (PubMed Search)
Posted: 6/23/2024 by Robert Flint, MD
(Updated: 2/1/2026)
Click here to contact Robert Flint, MD
Two recommendations from the recent GRACE 4 publication in Academic Emergency Medicine to consider:
1. Use phenobarbital along with benzodiazepines in patients with moderate to severe alcohol withdrawal. The evidence isn’t robust but is positive when compared to benzos alone.
2. Adults with alcohol use disorder can benefit from anti-craving medications such as naloxone and gabapentin at time of discharge.
Guidelines for Reasonable and Appropriate Care in the Emergency Department (GRACE-4): Alcohol use disorder and cannabinoid hyperemesis syndrome management in the emergency department Bjug Borgundvaag PhD, MD, CCFP(EM), Fernanda Bellolio MD, MSc, Isabelle Miles MD, Evan S. Schwarz MD, Sameer Sharif MD, MSc, Mark K. Su MD, MPH, Kevin Baumgartner MD, David B. Liss MD, Hasan Sheikh MD, MPA, Jody Vogel MD, MSc, MSW, Emily B. Austin MD, Suneel Upadhye MD, MSc, Michelle Klaiman MD, FRCPC, DABAM, Robert Vellend, Anna Munkley, Christopher R. Carpenter MD, MSc
First published: 15 May 2024
Academic Emergency Medicine https://doi.org/10.1111/acem.14911
Category: Pharmacology & Therapeutics
Keywords: Pharmacology, Toxicology, Acetaminophen, Acetylcysteine, NAC (PubMed Search)
Posted: 6/13/2024 by Matthew Poremba
Click here to contact Matthew Poremba
A panel comprised of 21 participants selected by four clinical toxicology societies (America’s Poison Centers, American Academy of Clinical Toxicology, American College of Medical Toxicology, and Canadian Association of Poison Control Centers) sought to develop consensus guidelines for management of acetaminophen poisoning in the US and Canada. Highlights from this framework include:
Acetylcysteine Stopping Criteria
A common misconception is that acetylcysteine is administered for 21 hours then discontinued. The consensus statement codifies the practice of reassessing the patient at the end of the acetylcysteine infusion and only stopping acetylcysteine if all of the following criteria are met:
Ingestion of Extended-Release Acetaminophen Products
Extended release acetaminophen products are available on the US market. Management is largely the same as for instant release acetaminophen except for several exceptions:
Management of Repeated Supratherapeutic Acetaminophen Ingestion
When a patient presents following repeated acetaminophen ingestions over a period of greater than 24 hours the Matthew-Romack nomogram is no longer applicable for guiding decisions regarding treatment with acetylcysteine. The consensus statement recommends initiating treatment in this scenario if the patient’s acetaminophen concentration is > 20 mcg/mL or if patient’s AST/ALT are abnormal.
Final Thoughts:
These guidelines will function as a useful reference and officially codify a general framework with evidence-based recommendations for the management of acetaminophen poisoning. As always, a poison center or clinical toxicologist should be consulted for any complicated or serious acetaminophen poisoning.
Dart, Richard C et al. “Management of Acetaminophen Poisoning in the US and Canada: A Consensus Statement.” JAMA network open vol. 6,8 e2327739. 1 Aug. 2023, doi:10.1001/jamanetworkopen.2023.27739
Category: Pharmacology & Therapeutics
Keywords: myasthenia gravis, myasthenic crisis, exacerbation, drugs to avoid (PubMed Search)
Posted: 5/9/2024 by Alicia Pycraft
Click here to contact Alicia Pycraft
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder that affects an estimated 14 to 20 patients per 100,000 in the United States. Most patients with MG have autoantibodies against acetylcholine receptors (AChRs), which disrupt neuromuscular transmission through downregulation, destruction, blocking of AChRs or disrupting receptors in the postsynaptic membrane.
Several medications may worsen MG or precipitate myasthenic crisis, however, incidence is difficult to describe as literature is largely limited to case reports and there is often presence of other confounding factors. There are two proposed mechanisms for medications to cause or exacerbate MG:
Several medications commonly used in the emergency department are known to impair neuromuscular transmission and may induce or worsen MG. The following medications should be avoided, or used with extreme caution in patients with MG*:

*This list contains several common medications utilized in the emergency department, but is not an all-inclusive list of medications that may exacerbate MG. Please refer to the reference section for additional information.
