Category: Critical Care
Keywords: sepsis, subtypes, long term mortality, disability (PubMed Search)
Posted: 1/20/2026 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Settings: Secondary analysis of the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial.
Participants:
1368 patients who survived on day 28 after enrollment, and were retrospectively assigned different subtypes:
Low risk, barriers to care. Younger patients with few comorbidities, less severe disease,
Unhealthy baseline with severe illness: Previously healthy with severe illness and complex needs after discharge, barriers to care.
Multimorbidity. Older patients with more comorbidities and are frequently readmitted.
Low functional status: Poor functional status. Older patients with high prevalence of frailty at discharge and high functional needs who are often discharged to a facility.
Unhealthy baseline with severe illness: Existing poor health with severe illness and complex needs after discharge. Older patients with severe comorbidities, more severe illness, high functional needs, prolonged hospital stay,
Outcome measurement:
A) 90-day mortality,
B) 6-month and 12-month EuroQol 5D five level score
Study Results:
A) 90-day mortality:
Unhealthy baseline with severe illness (37.6%) > low functional status (45.5%) > multimorbidity (17.4%) > unhealthy baseline, severe illness (13.2%) > Low risk (5.1%).
B) 6-month EuroQol 5D-Five Level: lower score, lower functional outcomes)
Unhealthy baseline with severe illness (0.53) > unhealthy baseline, severe illness (0.68) > low functional status (0.69) > multimorbidity (0.78) > Low risk (0.80).
Discussion:
a) The framework, readily available to clinicians provides good prognostic tools for mortality.
b) Although there was prediction of poor functional outcomes at 6-month and 12-month, the differences between subtypes in their EuroQoL 5D-5L did not seem to correspond to 90-day mortality. Low functional status group had 2nd-highest rate of mortality, but only 3rd in their EuroQoL 5D-3L score. Thus, there needs to be more studies in these nuances.
Conclusion:
Sepsis survivor subtypes—assigned using only three routinely available discharge variables—are strongly associated with 3-month mortality and long-term disability and HRQOL up to 12 months
Flick RJ, Kamphuis LA, Valley TS, Armstrong-Hough M, Iwashyna TJ. Association of Sepsis Survivor Subtypes With Long-Term Mortality and Disability After Discharge: A Retrospective Cohort Study. Crit Care Med. 2026 Jan 1;54(1):45-54. doi: 10.1097/CCM.0000000000006933. Epub 2025 Nov 13. PMID: 41231072.
Category: Critical Care
Keywords: critically ill, ED, boarding, outcome (PubMed Search)
Posted: 11/25/2025 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Settings: this is a meta-analysis of 17 observational studies about boarding of critically ill patients in US Emergency Departments. All studies were from urban, academic centers.
Participants:
Outcome measurement: all cause mortality, as reported by the authors of the original studies.
Study Results:
Discussion:
Conclusion:
Critically ill patients boarding in the U.S. Emergency Departments were associated with a non-statistically signi?cant increase in odds of mortality and hospital length of stay compared to non-boarded patients
Htet NN, Walker JA, Jafari D, Rech MA, Hintze T, Moran M, Bai J, Dinh K, Essaihi A, Wilairat S, Huddleson B, Tran QK. Outcomes of boarding critically ill patients in U.S. EDs: A systematic review and meta-analysis. Am J Emerg Med. 2025 Oct 17;99:339-347. doi: 10.1016/j.ajem.2025.10.036. Epub ahead of print. PMID: 41151219.
Category: Critical Care
Keywords: delirium, ICU, acetylcholinesterase inhibitor (PubMed Search)
Posted: 10/14/2025 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Delirium is common among critically ill patients. Some of the common Acetylcholinesterase inhibitors (AChEI), rivastigmine, donepezil, have been used to prevent delirium in ICU patients. However, their efficacy was just recently re-examined in a meta-analysis of only Randomized Control Trials.
Ten studies and 731 patients were included- 365 in the treatment (AChEI) group and 366 in the control group.
AChEI was associated with lower occurrence of delirium (RR 0.68, 95% CI 0.47-0.98, p=0.039. However, there was no significant difference in the delirium duration (mean difference -0.16 day, 95% CI -0.95 to 0.62 day, p=0.23). There was no difference in delirium severity nor length of hospital stay.
Among the medication, interestingly, rivastigmine 4.5 mg/day significantly reduced delirium occurrence (RR = 0.61 [0.39– 0.97]) and severity (SMD = –0.33 [–0.58 to –0.08]), as well as length of hospital stay (MD = –1.29 [–1.87 to –0.72]).
Discussion:
This meta-analysis was well-conducted.
The cholinergic dysregulation—especially elevated acetylcholinesterase activity—can lead to the imbalance between attention and cognition, contributing to delirium in ICU patients. Thus, the use of AChEI and reduction of occurrence of delirium proves that acetylcholine deficiency may be associated with delirium among ICU patients.
Subgroup analysis showed that prophylactic use of AChEI was associated with significant reduction of delirium duration. Thus, further studies are needed to define which populations will benefit from AChEI.
Conclusion:
AChEIs are effective in reducing occurrence of delirium, but they did not affect delirium duration, severity or hospital LOS.
Pipek LZ, Pascual GS, Nascimento RFV, Silva GD, Castro LH. Acetylcholinesterase Inhibitors for Delirium Prevention: A Systematic Review and Meta-Analysis. Crit Care Med. 2025 Oct 1;53(10):e2054-e2061. doi: 10.1097/CCM.0000000000006786. Epub 2025 Aug 5. PMID: 40758382.
Category: Critical Care
Keywords: sepsis, septic shock, omeprazole, proton pump inhibitor, anti-inflammatory (PubMed Search)
Posted: 9/30/2025 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Settings: multinational, randomized, double- blind, placebo-controlled clinical trial conducted in 17 centers in Italy, Russia, and Kazakhstan
Participants: A total of 307 ICU patients with sepsis or septic shock. Patients who were likely to die (APACHE II > 65 points) were excluded.
Treatment group: 80 mg bolus of omeprazole at randomization, at 12 hours and infusion of 12 mg/hour for 72 hours. Total dose of 1024 mg.
Outcome measurement: primary outcome of the study was organ dysfunction measured as the mean daily SOFA score during the first 10 days. Secondary outcomes were antibiotics-free days at 28 days; all-cause mortality at 28 days
Study Results:
Discussion:
Conclusion:
In sepsis patients, Esomeprazole did not re- duce organ dysfunction, despite demonstrating in vivo immunomodulatory effects
Monti G, Carta S, Kotani Y, Bruni A, Konkayeva M, Guarracino F, Yakovlev A, Cucciolini G, Shemetova M, Scapol S, Momesso E, Garofalo E, Brizzi G, Baldassarri R, Ajello S, Isirdi A, Meroi F, Baiardo Redaelli M, Boffa N, Votta CD, Borghi G, Montrucchio G, Rauch S, D'Amico F, Pace MC, Paternoster G, Vitale F, Giardina G, Labanca R, Lembo R, Marmiere M, Marzaroli M, Nakhnoukh C, Plumari V, Scandroglio AM, Scquizzato T, Sordoni S, Valsecchi D, Agrò FE, Finco G, Bove T, Corradi F, Likhvantsev V, Longhini F, Konkayev A, Landoni G, Bellomo R, Zangrillo A; PPI-SEPSIS Study Group. A Multinational Randomized Trial of Mega-Dose Esomeprazole as Anti-Inflammatory Agent in Sepsis. Crit Care Med. 2025 Aug 1;53(8):e1554-e1566. doi: 10.1097/CCM.0000000000006720. Epub 2025 May 29. PMID: 40439536.
Category: Critical Care
Keywords: diarrhea, ICU, mechanically ventilated (PubMed Search)
Posted: 8/4/2025 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Have you ever wondered what happened to your mechanically ventilated patients who developed diarrhea. Apparently, a multicenter study involving 2650 patients from 44 ICUs in the US, Canada and Saudi Arabia investigated the prevalence of diarrhea among these patients.
This study was the Editor’s choice for June 2025.
Results:
The mean age for the population was 59.8 (16.5) years, with APACHE II Score of 22.0 (7.8). Up to 61% of the patients received vasopressors or inotropes on day 1, which mean these patients are relatively ill.
Up to 60% of patients had diarrhea during their ICU stay, with 15% had diarrhea on day 1 or 2.
Initiating laxatives and antibiotics (who in the ICU would not receive vitamin V and Vitamin Z?) were associated with increased risk of diarrhea: HR for laxatives 1.28 (1.13–1.44), p<0.001; HR for antibiotics 1.41 (1.20–1.67), P< 0.001.
Furthermore, enteral feeding with high/moderate protein concentration was also associated with diarrhea (HR 1.13, 1.00-1.28, P=0.045.
Not surprisingly, diarrhea was associated with higher number of C. Diff testing.
Although patients with diarrhea were associated with longer ICU stay (15 [10-23] days) vs. those without diarrhea (8 [6-12] days), it was not associated with higher mortality (HR 0.70, 95% CI 0.57-0.86, P<0.001)
Discussion:
1. The authors did not report the rates of positive C. Diff. infection in these patients during ICU stay, although they did report that for another study in this population, the rate of positive C. Diff. infection during ICU stay was 2.2%. If only 2.2% had C. Diff. infection while up to 60% had diarrhea. Consequently, for every 30 patients with diarrhea, only one patient had C. Diff. infection. Therefore, do we have to check C. Diff. in those ICU patients with diarrhea every time?
2. The authors hypothesized that patients with diarrhea had longer ICU stay and lower mortality because they survived long enough to develop diarrhea. Thus, diarrhea is bad for clinicians, but may not be too bad for patients?
Conclusion:
Diarrhea is common among invasively ventilated patients. Patients who received laxatives, antibiotics, enteral feeding with high protein amount are at higher risk for diarrhea.
Dionne JC, Johnstone J, Heels-Ansdell D, Tahvildar Khazaneh P, Zytaruk N, Clarke F, Hand L, Millen T, Dechert W, Porteous R, Auld F, Hunt M, Campbell E, Bentall T, Campbell T, Smith O, Rose L, Arabi YM, Duan E, Wilcox ME, McIntyre L, Rochwerg B, Karachi T, Adhikari NK, Charbonney E, St-Arnaud C, Kristof A, Khwaja K, Marquis F, Zarychanski R, Golan E, Cook D; PROSPECT Research Coordinators Group, the PROSPECT Investigators and the Canadian Critical Care Trials Group. Diarrhea in Mechanically Ventilated Patients: A Nested Multicenter Substudy. Crit Care Med. 2025 Jun 1;53(6):e1247-e1256. doi: 10.1097/CCM.0000000000006667. Epub 2025 Apr 3. PMID: 40459385.
Category: Critical Care
Posted: 5/27/2025 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
We have known that resuscitation with balanced crystalloids was associated with better outcomes, than normal saline. However, I have believed that in the early phase of resuscitation, volume of any crystalloids is still better than little volume. Thus, a couple of liters of normal saline (0.9% saline) would not hurt. However, the recent secondary analysis from the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial might have changed my practice.
-----
Settings:
60 ICU in the United States between 2018 to 2022. This is the secondary analysis of the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial population
Participants: Patients with sepsis-induced hypotension after receiving fluid resuscitation (from 1-3 litters). Participants who received lactate ringers (622 patients) were compared with patients who received normal saline (690 patients).
Outcome measurement:
Death before discharge home by day 90.
Hospital-free days at 28 days
Study Results:
Dead occurred in 12.2% of LR group (76/622) vs. 15.9% (110/690) patients of the NS group, Adjusted Hazard Ratio 0.71 (95% CI 0.51-0.99, p=0.043)
Patients receiving LR had more hospital-free days at 28 days than those receiving 0.9% saline (16.6 ± 10.8 vs. 15.4 ± 11.4 d, respectively). The mean difference was 1.6 days (95% CI, 0.4–2.8; p = 0.009).
Discussion:
This study confirms that not only early resuscitation is important, but the fluid choice during the early resuscitation phase is also important, especially in patients with signs and symptoms of sepsis.
Thus, even during pre-hospital phase and in the ED, clinicians should consider to use LR or other balanced solutions if available.
Conclusion:
Among patients with sepsis-induced hypotension, resuscitation with Lactate Ringer was associated with better outcomes than normal saline.
Gelbenegger G, Shapiro NI, Zeitlinger M, Jilma B, Douglas IS, Jorda A. Lactated Ringer's or Normal Saline for Initial Fluid Resuscitation in Sepsis-Induced Hypotension. Crit Care Med. 2025 May 1;53(5):e1140-e1144. doi: 10.1097/CCM.0000000000006601. Epub 2025 Feb 19. PMID: 39969246; PMCID: PMC12047640.
Category: Critical Care
Keywords: community acquired pneumonia; CAP; corticosteroids; mortality; adjuvant therapy (PubMed Search)
Posted: 3/25/2025 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
If you watch those medical drama (House MD, ER, Grey’s Anatomy, Resident…), the doctors and residents are always faced with a dilemma – is it a rare autoimmune disorder or is it an infection? They are worried that if they give steroid to a patient with infections, that would kill the patients.
Well, it might not be the case for Community acquired pneumonia.
A meta-analysis of randomized control trials involving 3224 patients to look into the efficacy of adjuvant corticosteroids for CAP. The authors assessed the heterogeneity of treatment effect (different groups should have different response to treatment).
For patients who were anticipated to benefit (those who had CRP > 240 mg/L), corticosteroids were associated with lower odds of 30-day mortality (OR 0·43 [0·25–0·76], p=0·026).
When stratifying by risk, there was no significant effect between those with Pneumonia Severity Index (PSI) I-III versus those with PSI IV-V.
However, corticosteroids increased odds of hyperglycemia (OR 2·50 [95% CI 1·63–3·83], p<0·0001), odds of hospital readmissions (1·95 [1·24–3·07], p=0·0038)
Discussion:
There were different regiments for corticosteroids in the included studies. However, hydrocortisone appeared to be more effective than other corticosteroids.
Furthermore, the time intervals for treatment is still debatable. The data suggested that the ideal treatment is within 24 hours of hospital admission, but patients can still benefit from treatment in up to 48 hours.
A response-dependent treatment is also recommended: 8 days or 14 days, depending on how patients respond to treatment by day 4.
Conclusion:
Adjuvant treatment with corticosteroids among hospitalized patients with CAP was significantly associated with reduction of 30-day mortality. The treatment effect, however, varied according to patients CRP concentrations at baseline.
Smit JM, Van Der Zee PA, Stoof SCM, Van Genderen ME, Snijders D, Boersma WG, Confalonieri P, Salton F, Confalonieri M, Shih MC, Meduri GU, Dequin PF, Le Gouge A, Lloyd M, Karunajeewa H, Bartminski G, Fernández-Serrano S, Suárez-Cuartín G, van Klaveren D, Briel M, Schönenberger CM, Steyerberg EW, Gommers DAMPJ, Bax HI, Bos WJW, van de Garde EMW, Wittermans E, Grutters JC, Blum CA, Christ-Crain M, Torres A, Motos A, Reinders MJT, Van Bommel J, Krijthe JH, Endeman H. Predicting benefit from adjuvant therapy with corticosteroids in community-acquired pneumonia: a data-driven analysis of randomised trials. Lancet Respir Med. 2025 Mar;13(3):221-233. doi: 10.1016/S2213-2600(24)00405-3. Epub 2025 Jan 29. PMID: 39892408.
Category: Critical Care
Keywords: delirium, ICU, window (PubMed Search)
Posted: 1/21/2025 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Delirium in the ICU means badness as delirious ICU patients are associated with longer stay and higher mortality. While medications are not proven to prevent delirium, certain environmental interventions such as window access, light and sound levels have been recognized as legit interventions to prevent ICU delirium.
Settings: This is a retrospective study at Massachusetts General Hospital
Participants: 3527 patients admitted to a surgical ICU between 2020 and 2023.
Outcome measurement: This study hypothesized that patients in a windowed ICU room will have lower rates of delirium, decreased ICU length of stay, hospital LOS. Multivariable logistic regressions were performed for the association of clinical variables and the presence of delirium.
Study Results:
Delirium was observed in 460 patients (21%) of the windowed rooms group and 206 patients (16%) of the nonwindowed rooms group. Multivariable logistic regression showed that patients in windowed rooms were associated with higher odds of delirium (aOR, 1.29; 95% CI, 1.07–1.56; p = 0.008), although they were not associated with longer ICU LOS or longer HLOS
Discussion:
The study’s findings added to the literature that natural lighting might not be the effective prevention of delirium. The presence of windows might not be the answer.
In this study, all the windows were facing another building, and there was no view of other natural scenes, with a limited view of the sky. Therefore, the authors suggested that the overall quality of the windows would be more important.
Conclusion:
The ICU environment is more important for patients’ delirium than just the presence of windows.
Anderson DC, Warner PE, Smith MR, Albanese ML, Mueller AL, Messervy J, Renne BC, Smith SJ. Windows in the ICU and Postoperative Delirium: A Retrospective Cohort Study. Crit Care Med. 2025 Jan 13. doi: 10.1097/CCM.0000000000006557. Epub ahead of print. PMID: 39791968.
Category: Critical Care
Keywords: ketamine, etomidate, rapid sequence intubation, hemodynamic instability, adrenal suppression (PubMed Search)
Posted: 11/26/2024 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
It’s the age-old question. We’ve read studies comparing propofol vs. etomidate, ketofol vs. etomidate, and now a meta-analysis about ketamine vs. etomidate. Etomidate is the staple induction agent for RSI, mostly used by Emergency Medicine, and to a degree in the Intensive Care Unit. However, the question about adrenal suppression was initiated in the early 2000s and researchers have been looking for other alternatives. This meta analysis attempted to look for another answer.
Settings: A meta-analysis of randomized controlled trials
Participants: 2384 patients who needed emergent intubation were included.
Outcome measurement: Peri-intubation instability
Study Results:
Compared with etomidate, ketamine was associated with higher risk of hemodynamic instability and moderate certainty (RR 1.29, 95% CI 1.07-1.57).
Ketamine was associated with lower risk of adrenal suppression, again, with moderate uncertainty (RR 0.54, 95% CI 0.45-0.66).
Ketamine was not associated with differences and risk of first successful intubation nor mortality.
Discussion:
Most studies were single center and involved small-moderate sample size, ranging from 20 patients to 700 patients.
For adrenal suppression, there were only 3 studies and a total of 1280 patients, thus, the results are still not definitive.
For an academic exercise, the Number Needed to Harm for both hemodynamic instability and adrenal suppression are calculated here.
Number Needed to Harm for hemodynamic instability: 25.
Number needed to harm for adrendal suppression: 11.
Greer A, Hewitt M, Khazaneh PT, Ergan B, Burry L, Semler MW, Rochwerg B, Sharif S. Ketamine Versus Etomidate for Rapid Sequence Intubation: A Systematic Review and Meta-Analysis of Randomized Trials
Category: Critical Care
Keywords: albumin, crystalloid, septic shock, mortality (PubMed Search)
Posted: 10/1/2024 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Title: Albumin Versus Balanced Crystalloid for the Early Resuscitation of Sepsis: An Open Parallel-Group Randomized Feasibility Trial— The ABC-Sepsis Trial
Settings: 15 ED in the United Kingdom. This study is a feasibility study but it looked at mortality as a primary outcome.
Participants:
• Patients with Sepsis, with their National Early Warning Score (NEWS) ? 5 (These patients have estimated mortality of 20%). IV fluid resuscitation needs to be within 1 hour of assessment.
• 300 Patients were randomized to receive balanced crystalloids or 5% human albumin solution (HAS) only, within 6 hours of randomization.
Outcome measurement: 30-day mortality, Hospital length of stay (HLOS)
Study Results:
• The median time for receiving IV fluid from randomization was 41 minutes (HAS) vs. 36 minutes (crystalloids).
• Total volume of IV fluid per Kg in first 6 hours 14.5 ml/kg (HAS) vs. 18.8 ml/kg (crystalloids).
• Other interventions (vasopressor, Renal replacement therapy, invasive ventilation) were similar.
• Complications (AKI, pulmonary edema, allergy) were lower for Crystalloids group
• Median hospital LOS = 6 days for both groups.
• 90-day mortality: 31 (21.1%) (HAS) vs. 22 (14.8%) (Crystalloids), OR 1.54 (95% 0.8-2.8)
Discussion:
• Total volumes for resuscitation in the first 6 hours was 750 ml (HAS) and 1250 ml (crystalloids). This signified a trend toward lower total volume of resuscitation (remember that 30 ml/kg recommendation)
• The 2024 guidelines from Chest (REF 2) suggested that: “In Critically ill adult patients (excluding patients with thermal injuries and ARDS), intravenous albumin is not suggested for first line volume replacement or to increase serum albumin levels. Therefore, we should not give patients (except for cirrhosis or spontaneous bacterial peritonitis) albumin just to reduce the volume of fluid.
• The authors suggested that even a definitive trial in the future will not be able to demonstrate a significant benefit of using 5% albumin.
Conclusion:
There is lower mortality (numerical but not statistically) among the group with balanced crystalloids.
1. Gray AJ, Oatey K, Grahamslaw J, Irvine S, Cafferkey J, Kennel T, Norrie J, Walsh T, Lone N, Horner D, Appelboam A, Hall P, Skipworth RJE, Bell D, Rooney K, Shankar-Hari M, Corfield AR; Albumin, Balanced, and Crystalloid-Sepsis (ABC-Sepsis) Investigators. Albumin Versus Balanced Crystalloid for the Early Resuscitation of Sepsis: An Open Parallel-Group Randomized Feasibility Trial- The ABC-Sepsis Trial. Crit Care Med. 2024 Oct 1;52(10):1520-1532. doi: 10.1097/CCM.0000000000006348. Epub 2024 Jun 24. PMID: 38912884.
2. Callum J, Skubas NJ, Bathla A, Keshavarz H, Clark EG, Rochwerg B, Fergusson D, Arbous S, Bauer SR, China L, Fung M, Jug R, Neill M, Paine C, Pavenski K, Shah PS, Robinson S, Shan H, Szczepiorkowski ZM, Thevenot T, Wu B, Stanworth S, Shehata N; International Collaboration for Transfusion Medicine Guidelines Intravenous Albumin Guideline Group. Use of Intravenous Albumin: A Guideline From the International Collaboration for Transfusion Medicine Guidelines. Chest. 2024 Aug;166(2):321-338. doi: 10.1016/j.chest.2024.02.049. Epub 2024 Mar 4. PMID: 38447639; PMCID: PMC11317816.
Category: Critical Care
Keywords: meropenem, continuous administration, critically ill (PubMed Search)
Posted: 8/6/2024 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
We heard it before. Continuous administration of antibiotics might be associated with better outcomes. However, results from smaller randomized controlled trials of beta-lactam showed inconsistent conclusions. Therefore, a large RCT was conducted
Settings: 31 ICUs in Croatia, Italy, Kazakhstan, Russia between June 2018 – August 2022.
Randomized, double-blind control trial.
Participants:
Outcome measurement:
Study Results:
Discussion:
Conclusion:
In critically ill patients with sepsis, continuous administration of meropenem did not improve mortality nor reduce the emergence of pandrug resistant bacteria.
Monti G, Bradic N, Marzaroli M, Konkayev A, Fominskiy E, Kotani Y, Likhvantsev VV, Momesso E, Nogtev P, Lobreglio R, Redkin I, Toffoletto F, Bruni A, Baiardo Redaelli M, D'Andrea N, Paternoster G, Scandroglio AM, Gallicchio F, Ballestra M, Calabrò MG, Cotoia A, Perone R, Cuffaro R, Montrucchio G, Pota V, Ananiadou S, Lembo R, Musu M, Rauch S, Galbiati C, Pinelli F, Pasin L, Guarracino F, Santarpino G, Agrò FE, Bove T, Corradi F, Forfori F, Longhini F, Cecconi M, Landoni G, Bellomo R, Zangrillo A; MERCY Investigators. Continuous vs Intermittent Meropenem Administration in Critically Ill Patients With Sepsis: The MERCY Randomized Clinical Trial. JAMA. 2023 Jul 11;330(2):141-151. doi: 10.1001/jama.2023.10598. PMID: 37326473; PMCID: PMC10276329.
Category: Critical Care
Keywords: ICU, delirium, antipsychotic (PubMed Search)
Posted: 6/18/2024 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Title: Antipsychotics in the Treatment of Delirium in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
We all do it. When our patients in the ICU develop delirium, we would give them an antipsychotic, commonly quetiapine (Brand name Seroquel), and all is good. However, results from this most recent meta-analysis may suggest otherwise.
Settings: This is a meta-analysis from 5 Randomized Control Trials. Intervention was antipsychotic vs. placebo or just standard of care.
Participants: The 5 trials included A total of 1750 participants. All trials used Confusion Assessment Method for the ICU or Intensive Care Delirium Screening Checklist to measure delirium.
Outcome measurement: Delirium – and Coma-Free days
Study Results:
The use of any antipsychotic (typical or atypical) did not result in a statistically significant difference in delirium- and coma-free days among patients with ICU delirium (Mean Difference of 0.9 day; 95% CI -0.32 to 2.12).
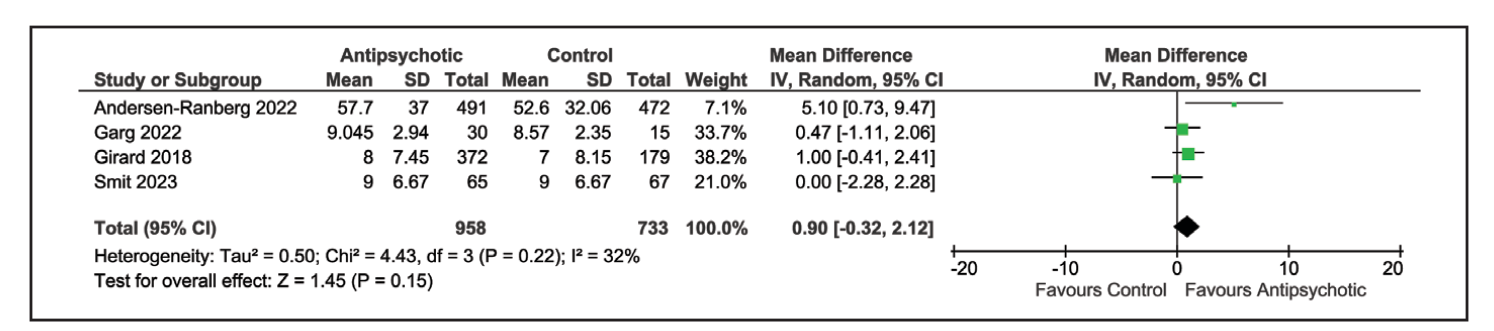
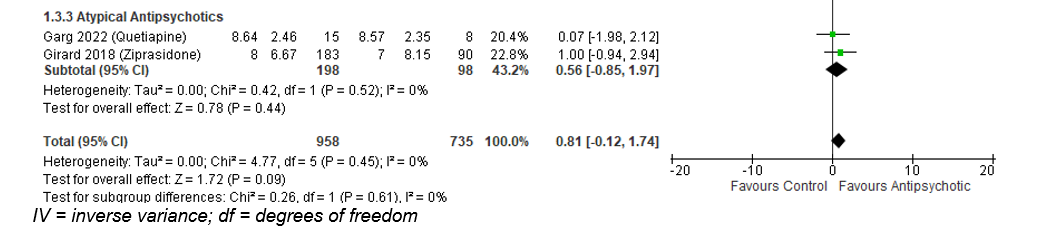
Similarly, atypical antipsychotic medication also did not result in difference of delirium- and coma-free days: Mean difference of 0.56 day; 95% CI -0.85 to 1.97).
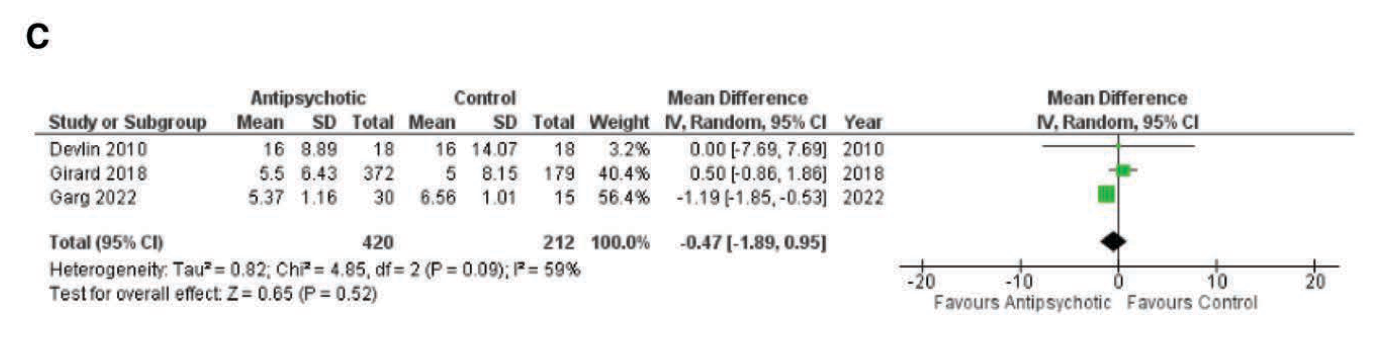
ICU length of stay was also not different in the group receiving antipsychotic: Mean difference -0.47 day, 95% CI -1.89 to 0.95).
Discussion:
The authors used both delirium -free and coma-free days as a composite outcome because they reasoned that delirium cannot be evaluated in unresponsive patients. This composite outcome might have affected the true incidence of delirium and the outcome of delirium-free days.
This meta-analysis would be different from previous ones that aimed to answer the same question. Previous studies compared either haloperidol vs a broader range of other medication (atypical antipsychotic, benzodiazepines) (Reference 2) or included all ICU patients with or without delirium who received haloperidol vs. placebo (Reference 3). Overall, those previous studies also reported that the use of haloperidol has not resulted in improvement of delirium-free days.
Conclusion:
There is evidence that the use of anti-psychotic medication does not result in difference of delirium- or coma-free days among critically ill patients with delirium.
1.Carayannopoulos KL, Alshamsi F, Chaudhuri D, Spatafora L, Piticaru J, Campbell K, Alhazzani W, Lewis K. Antipsychotics in the Treatment of Delirium in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Med. 2024 Jul 1;52(7):1087-1096. doi: 10.1097/CCM.0000000000006251. Epub 2024 Mar 15. PMID: 38488422.
2. Andersen-Ranberg NC, Barbateskovic M, Perner A, Oxenbøll Collet M, Musaeus Poulsen L, van der Jagt M, Smit L, Wetterslev J, Mathiesen O, Maagaard M. Haloperidol for the treatment of delirium in critically ill patients: an updated systematic review with meta-analysis and trial sequential analysis. Crit Care. 2023 Aug 26;27(1):329. doi: 10.1186/s13054-023-04621-4. PMID: 37633991; PMCID: PMC10463604.
3. Huang J, Zheng H, Zhu X, Zhang K, Ping X. The efficacy and safety of haloperidol for the treatment of delirium in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2023 Jul 27;10:1200314. doi: 10.3389/fmed.2023.1200314. PMID: 37575982; PMCID: PMC10414537.
Category: Critical Care
Posted: 4/30/2024 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Title: Safety and Efficacy of Reduced-Dose Versus Full-Dose Alteplase for Acute Pulmonary Embolism: A Multicenter Observational Comparative Effectiveness Study
Settings: Retrospective observational study from a combination of Abbott Northwestern Hospital and 15 others as part of the Mayo Health system.
Participants: Patients between 2012 – 2020 who were treated for PE. Patients were propensity-matched according to the probability of a patient receiving a reduced- dose of alteplase.
Outcome measurement:
Study Results:
Discussion:
Conclusion:
In this retrospective, Propensity-score matching study, the full-dose regimen but is associated with a lower risk of bleeding.
Melamed R, Tierney DM, Xia R, Brown CS, Mara KC, Lillyblad M, Sidebottom A, Wiley BM, Khapov I, Gajic O. Safety and Efficacy of Reduced-Dose Versus Full-Dose Alteplase for Acute Pulmonary Embolism: A Multicenter Observational Comparative Effectiveness Study. Crit Care Med. 2024 May 1;52(5):729-742. doi: 10.1097/CCM.0000000000006162. Epub 2024 Jan 3. PMID: 38165776.
Category: Critical Care
Posted: 3/12/2024 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Background: There is no clear guidelines regarding whether norepinephrine or epinephrine would be the preferred agent to maintain hemodynamic stability after cardiac arrest. In recent years, there has been more opinions about the use of norepinephrine in this situation.
Settings: retrospective multi-site cohort study of adult patients who presented to emergency departments at Mayo Clinic hospitals in Minnesota, Florida, Arizona with out-of-hospital-cardiac arrest (OHCA). Study period was May 5th, 2018, to January 31st, 2022
Participants: 18 years of age and older
Outcome measurement: tachycardia, rate of re-arrest during hospitalization, in-hospital mortality.
Multivariate logistic regressions were performed.
Study Results:
Discussion:
It was retrospective study that uses electronic health records. Thus, other important factors from the pre-hospital settings might not be accurate.
On the other hand, the patient population came from multiple hospitals with varying practices so the patient population is more generalizable.
Conclusion:
Although the rate of tachyarrhythmia was not different between patients receiving norepinephrine vs. epinephrine after ROSC. This study would add more data to the current literature that norepinephrine might be more beneficial for patients with post-cardiac arrest shock.
Normand S, Matthews C, Brown CS, Mattson AE, Mara KC, Bellolio F, Wieruszewski ED. Risk of arrhythmia in post-resuscitative shock after out-of-hospital cardiac arrest with epinephrine versus norepinephrine. Am J Emerg Med. 2024 Mar;77:72-76. doi: 10.1016/j.ajem.2023.12.003. Epub 2023 Dec 10. PMID: 38104386.
Category: Critical Care
Keywords: OHCA, elevated head and thorax, chest compression (PubMed Search)
Posted: 1/23/2024 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Hot of the press from the Society of Critical Care Medicine (But most of us would know it already)
Settings: This is a prospective observational population-based study design with non-contemporaneous, nonrandomized clinical trial direct (unadjusted) head- to-head evaluations
Propensity score–matched comparisons of non-shockable cardiac arrest (NS-OHCA) patient survivor using conventional CPR (C-CPR) vs. C-CPR plus Automated Head/thorax up positioning-CPR (AHUP-CPR).
Participants: patients with non-traumatic, non-shockable out of hospital cardiac arrest (NS-OHCA).
Outcome measurement: primary outcome = survival, secondary outcome = survival with good neurologic outcome (Cerebral Performance Category score of 1–2 or modified Rankin Score less than or equal to 3).
Study Results:
• There was a total of 380 AHUP-CPR vs. 1852 C-CPR patients. After 1:1 matching, there were 353 AHUP-CPR patients and 353 C-CPR patients.
• In unadjusted analysis
o AHUP-CPR was associated with higher odds of survival (Odds ratio 2.46, 95% CI 1.55-3.92) and higher odds of survival with good neurologic function (Odds ratio 3.09 (95% CI 1.64-5.81)
• In matched groups
o AHUP-CPR was associated with higher odds of survival (Odds ratio 2.84, 95% CI 1.35-5.96) and higher odds of survival with good neurologic function [Odds ratio 3.87 (95% CI 11.27-11.78]
Discussion:
• There was no difference in rates of ROSC between groups. The authors argued that there was “neuroprotective effects” for the AHUP-CPR group.
• Although randomized controlled trials are usually required before clinical interventions are adopted, the aurthors argued that it would be difficult to randomize OHCA patients, and that the risk vs benefits may facilitate early adoption of this strategy.
• AHUP-CPR should be used first by well-trained clinicians to ensure its benefits.
Conclusion:
OHCA patients with NS presentations will have a much higher likelihood of surviving with good neurologic function when chest compressions are augmented by expedient application of the noninvasive tools to elevated head and thorax used in this study.
Bachista KM, Moore JC, Labarère J, Crowe RP, Emanuelson LD, Lick CJ, Debaty GP, Holley JE, Quinn RP, Scheppke KA, Pepe PE. Survival for Nonshockable Cardiac Arrests Treated With Noninvasive Circulatory Adjuncts and Head/Thorax Elevation. Crit Care Med. 2024 Feb 1;52(2):170-181. doi: 10.1097/CCM.0000000000006055. Epub 2024 Jan 19. PMID: 38240504.
Category: Critical Care
Keywords: vasopressor, norepinephrine, timing, septic shock (PubMed Search)
Posted: 12/5/2023 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: systemic review and meta-analysis
Participants: 2 RCTs, 21 observational studies. Fifteen studies were published between 2020-2023.
There was a total of 25721 patients with septic shock
Outcome measurement: Primary outcome was short-term mortality (ICU, hospital, 28-day, 30-day). Secondary outcomes included ICU LOS, Hospital LOS, time to achieve MAP > 65 mm Hg,
Study Results:
Composite outcome of short term mortality:
Secondary outcome:
Discussion:
Conclusion:
More and more studies, although a RCT is still necessary, are showing that early initiation of vasopressor within 1-6 hours of septic shock would be more beneficial to patients with septic shock.
Ye E, Ye H, Wang S, Fang X. INITIATION TIMING OF VASOPRESSOR IN PATIENTS WITH SEPTIC SHOCK: A SYSTEMATIC REVIEW AND META-ANALYSIS. Shock. 2023 Nov 1;60(5):627-636. doi: 10.1097/SHK.0000000000002214. Epub 2023 Sep 2. PMID: 37695641.
Category: Critical Care
Keywords: SOFA, admission unit, ICU, IMC, Ward, morality (PubMed Search)
Posted: 10/17/2023 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: Retrospective study of a national inpatient database (Japan).
Participants:
Outcome measurement: Primary outcome was in-hospital mortality, after propensity score matching.
Study Results:
Discussion:
Conclusion:
Risk-stratifying patients according to SOFA score is a potential strategy for appropriate admission strategies.
1.Ohbe H, Sasabuchi Y, Doi K, Matsui H, Yasunaga H. Association Between Levels of Intensive Care and In-Hospital Mortality in Patients Hospitalized for Sepsis Stratified by Sequential Organ Failure Assessment Scores. Crit Care Med. 2023 Sep 1;51(9):1138-1147. doi: 10.1097/CCM.0000000000005886. Epub 2023 Apr 28. PMID: 37114933.
2.Corwin GS, Mills PD, Shanawani H, Hemphill RR. Root Cause Analysis of ICU Adverse Events in the Veterans Health Administration. Jt Comm J Qual Patient Saf. 2017 Nov;43(11):580-590. doi: 10.1016/j.jcjq.2017.04.009. Epub 2017 Jul 25. PMID: 29056178.
Category: Critical Care
Keywords: arterial cannulation, axillary artery, femoral artery, infraclavicular (PubMed Search)
Posted: 8/21/2023 by Quincy Tran, MD, PhD
Click here to contact Quincy Tran, MD, PhD
Settings: Single ICU in Poland, randomized trial
Participants: intubated patients who needed arterial catheter placement. Patients who had adequate access to one axillary and one femoral artery were eligible.
Patients were randomized 1:1 for axillary or femoral artery cannulation.
Outcome measurement: Primary outcome was cannulation success rate. Secondary outcomes were first pass success rate, number of attempts.
Study Results:
Discussion:
Conclusion:
Ultrasound-guided cannulation of the axillary artery via the infraclavicular route is non-inferior to the cannulation of the common femoral artery. When cannulation of the radial or femoral artery is not available, we can consider axillary artery via the infraclavicular approach.
Reference:
Gawda, Ryszard MD, PhD; Marszalski, Maciej MD; Piwoda, Maciej MD; Molsa, Maciej MD; Pietka, Marek MD; Filipiak, Kamil MD; Miechowicz, Izabela PhD; Czarnik, Tomasz MD, PhD1. Infraclavicular, Ultrasound-Guided Percutaneous Approach to the Axillary Artery for Arterial Catheter Placement: A Randomized Trial. Critical Care Medicine ():10.1097/CCM.0000000000006015, August 07, 2023. | DOI: 10.1097/CCM.0000000000006015
Category: Critical Care
Keywords: NEWS, MEWS, IEWS, international Early Warning Score, mortality (PubMed Search)
Posted: 6/27/2023 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
Settings: Retrospective data from 3 Dutch EDs (development of the score), 2 Denmark ED (for validation of the score). The novel score (International Early Warning Score) will be composed of the National Early Warning Score (NEWS) + Age +Sex
Components of the National Early Warning Score:
Participants: All adult patients in the Netherlands Emergency department Evaluation Database (NEED) and Danish Multicenter Cohort (DMC).
Outcome measurement: in-hospital mortality, including death in EDs.
Study Results:
Discussion:
Conclusion:
This multicenter study showed that IEWS perform better than the NEWS for predicting in-hospital mortality for ED patients.
Candel BGJ, Nissen SK, Nickel CH, Raven W, Thijssen W, Gaakeer MI, Lassen AT, Brabrand M, Steyerberg EW, de Jonge E, de Groot B. Development and External Validation of the International Early Warning Score for Improved Age- and Sex-Adjusted In-Hospital Mortality Prediction in the Emergency Department. Crit Care Med. 2023 Jul 1;51(7):881-891. doi: 10.1097/CCM.0000000000005842. Epub 2023 Mar 23. PMID: 36951452; PMCID: PMC10262984.
Category: Critical Care
Keywords: etomidate, intubation, critically ill, mortality (PubMed Search)
Posted: 5/2/2023 by Quincy Tran, MD, PhD
(Updated: 2/1/2026)
Click here to contact Quincy Tran, MD, PhD
As emergency physicians, we use etomidate to intubate patients most of the time, although there was controversy whether etomidate would suppress critically ill patients’ cortisol production. Whether etomidate was associated with mortality was controversial. A recent meta-analysis investigated the issue again.
Methods: meta-analysis of randomized trials using etomidate for intubation versus other agents. Outcome = mortality as defined by the authors. Mortality was defined from 24 hours to 30 days by study’s authors.
Results: 11 RCTs, including one new RCT in 2022
319 (1359, 23%) patients received etomidate died vs. 267 (1345, 20%) receiving other agents died; Risk Ratio 1.16, 95% CI 1.01-1.33, P = 0.03.
Etomidate was also associated with higher risk ratio for adrenal insufficiency, when compared with other control agents (147/695, 21% vs. 69/686, 10%, RR 2.01, 95% CI (1.59-2.56), P < 0.01.
Etomidate was also associated with higher risk ratio of mortality, when compared with ketamine, for mortality, as defined by each study’s author (273/1201, 23% vs. 226/1198. 19%. RR 1.18, 95% CI 1.02-1.37, P = 0.03).
Discussion:
The authors used fixed effects model, as they claimed that their meta-analysis had low heterogeneity (I2 =0%). However, fixed effects model should only be used when there is no difference among patient population. In this study, the outcome definitions were different, the patient populations were different (trauma, pre-hospital, ED, ICU). Therefore, random effects model should be used. Random effects models tend to yield larger 95% CI, thus, more likely yield non-statistically significant results.
The authors claimed a Number Needed To Treat (NNT) for etomidate of 31, so basically many ED patients would die, while most of patients being intubated by Anesthesiology, regarding settings, would not die, as anesthesiologists mostly use propofol.
