Category: Critical Care
Posted: 1/13/2026 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Recall that MAP = (cardiac output) x (systemic vascular resistance)
Consequently, a patient can be normotensive due to increased SVR despite a very low cardiac output and shock. In fact, normotensive shock may have worse outcomes compared to patients with isolated hypotension.
Take home points:
Yerasi C, Case BC, Pahuja M, et al. "The Need for Additional Phenotyping When Defining Cardiogenic Shock." JACC Cardiovasc Interv. 2022;15(8):890-895.
Category: Critical Care
Posted: 11/18/2025 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
This is an actual patient case:
65 y/o pt intubated for hemoptysis and started on nebulized transexamic acid. Overnight, the pt is found to have severe breath stacking/auto-PEEPing and consequently is started on neuromuscular blockade. The pt has no history of asthma or COPD and the ETT is clear without obstruction.
Ventilator waveforms are as shown. What is the issue?
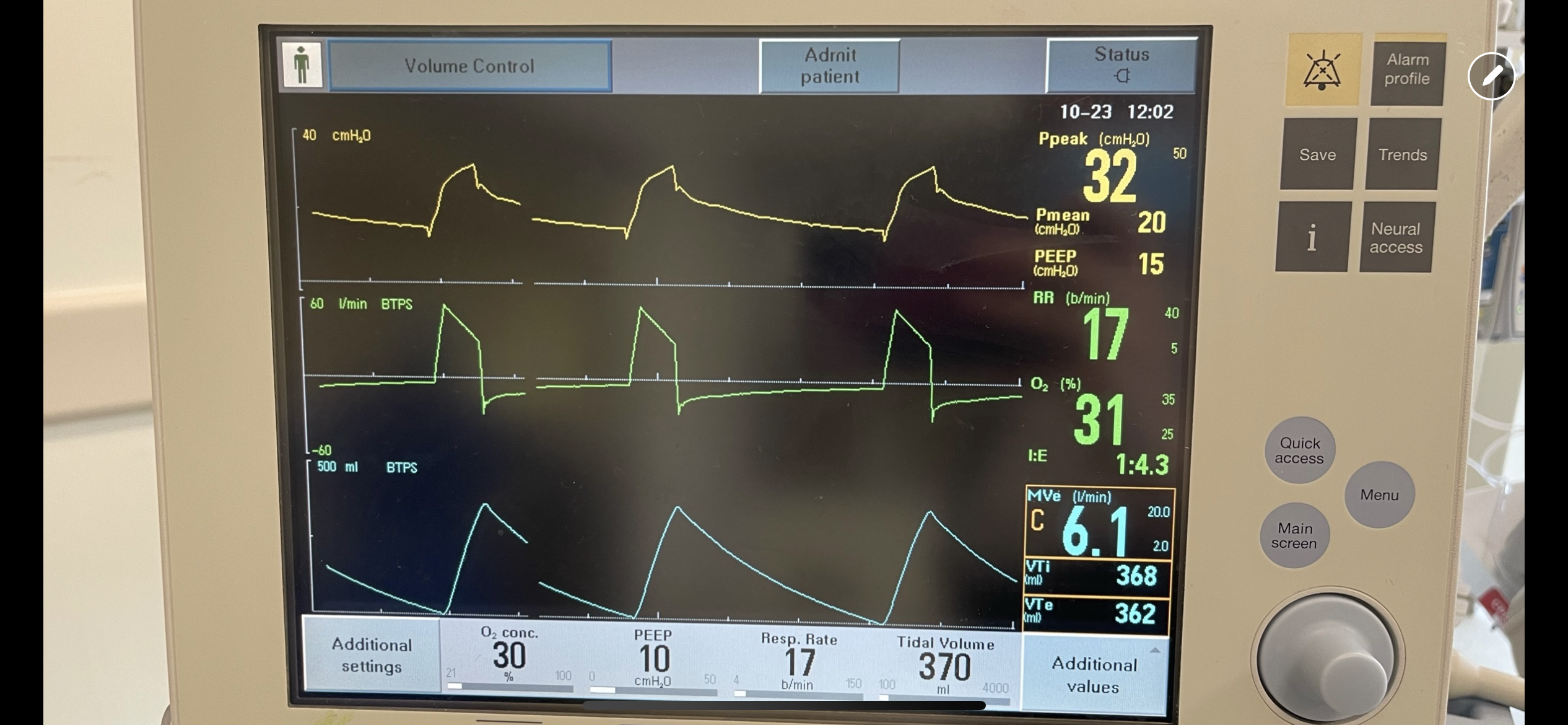
Explanation:
On expiration, the ventilator pressure (and the pressure curve waveform on the ventilator) should drop to the set PEEP (10 cm H2O in this case) immediately. This is true regardless of whether it is volume control, pressure control, PRVC etc. For this patient, the pressure curve is not dropping to the set PEEP immediately on expiration, rather, it slowly decays and does not even reach the set PEEP before the beginning of the next breath. This is not due to a patient issue, but rather an obstruction at the level of the ventilator. In particular, an obstruction in the expiratory limb of the tubing where flow returns to the ventilator from the patient. TXA is known to crystallize on the expiratory filter which can cause this type of obstruction if it is not changed frequently enough, preventing the pressure from dropping to PEEP and the patient from fully exhaling.
In this case, the obstruction was localized to the expiratory filter based on the ventilator waveforms and the filter was exchanged. The waveforms normalized, the patient had no obstruction or breath stacking, the neuromuscular blockade discontinued, and the patient was subsequently extubated without issue.
Category: Critical Care
Posted: 7/29/2025 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
PEEP is often titrated up along with FiO2 to increase oxygen saturation. Although the potential negative hemodynamic effect of high PEEP is often recognized, it is important to also note that high PEEP can also paradoxically worsen oxygen saturation.
The primary physiologic explanation for this phenomenon in a patient with pulmonary disease is due to the varying impact of PEEP on the intra- vs. extra-alveolar blood vessels. PEEP preferentially distends more normal/compliant lung which causes compression of intra-alveolar vessel at excessively high levels of PEEP. This causes pulmonary blood to be diverted to areas of lower vascular resistance (e.g. consolidated lung which is less distended due to its worsened compliance) and lower VQ matching. Essentially, blood flow to normal/healthy lung is decreased and is instead increased to diseased lung, worsening hypoxemia.
Bottom line:
High PEEP can potentially worsen hypoxemia and should be considered as an etiology for worsening oxygen saturation, particularly when the hypoxemia is out of proportion to the patient’s radiographic findings.
Çoruh B, Luks AM. Positive end-expiratory pressure. When more may not be better. Annals ATS. 2014;11(8):1327-1331.
Category: Critical Care
Posted: 5/21/2025 by Caleb Chan, MD
(Updated: 2/1/2026)
Click here to contact Caleb Chan, MD
DeMasi et al. published a review on the current evidence surrounding peri-intubation and intubation practices. While the actual approach and context to each patient will be different it is good to be aware of the actual evidence base for medical decision-making.
Preoxygenation
Between Induction and Laryngoscopy
During Laryngoscopy and Intubation of the Trachea
Medications
Interventions to Prevent Hypotension
DeMasi SC, Casey JD, Semler MW. Evidence-based emergency tracheal intubation. Am J Respir Crit Care Med. Published online April 16, 2025.
Category: Critical Care
Posted: 1/14/2025 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
These 2 papers challenge management dogmas in critical care that have persisted despite low-quality/absent evidence.
In particular, one explores the dogma, “bicarbonate improves ventricular contractility in severe metabolic acidosis,” with the following points:
-intracellular pH (which has a large impact on myocardial contractility) correlates poorly with blood gas pH
-many of the studies regarding bicarbonate in severe metabolic acidosis and hemodynamics are done on animal shock models
-two studies in patients with lactic acidosis showed increase in pH with bicarb administration without beneficial impact on hemodynamics (even in pts with pH < 7.1)
-bicarb administration is associated with hypernatremia, hypokalemia, and decreased ionized calcium levels
Hofmaenner DA, Singer M. Challenging management dogma where evidence is non-existent, weak or outdated. Intensive Care Med. 2022;48(5):548-558.
Hofmaenner DA, Singer M. Challenging management dogma where evidence is non-existent, weak, or outdated: part II. Intensive Care Med. 2024;50(11):1804-1813.
Category: Critical Care
Posted: 9/24/2024 by Caleb Chan, MD
(Updated: 2/1/2026)
Click here to contact Caleb Chan, MD
Some points from this narrative review:
Take home pearls:
van Eijk JA, Doeleman LC, Loer SA, Koster RW, van Schuppen H, Schober P. Ventilation during cardiopulmonary resuscitation: A narrative review. Resuscitation. 2024;203:110366.
Category: Critical Care
Posted: 6/11/2024 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Many patients present to the ED with hypercarbic respiratory failure (i.e. COPD exacerbation, obesity hypoventilation syndrome etc.). Typically, our first line of treatment is the use of BiPAP, where we set an inspiratory pressure (IPAP) and an expiratory pressure (EPAP). The difference between these two pressures (as well as patient effort) determines the tidal volumes (and consequently minute ventilation) a patient receives in our attempts to help the patient “blow off CO2.”
If you are having trouble with continued hypercarbia despite the use of BiPAP, another NIPPV mode that can be trialed is Average Volume-Assured Pressure Support (AVAPS). With BiPAP the patient receives the same fixed IPAP no matter what, even if their tidal volumes are lower than what is needed. With AVAPS, the ventilator will self-titrate the IPAP and increase (or decrease) the IPAP to reach the tidal volumes that you set, increasing the odds the patient will achieve the minute ventilation you are trying to achieve.
(AVAPS is essentially a non-invasive version of PRVC)
Initial settings (ask your RTs for help!):
Category: Critical Care
Posted: 4/23/2024 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Background:
-Muscle rigidity has been described as a side-effect of fentanyl, specifically activation of expiratory muscles
-Excessive expiratory muscle use acts as “anti-PEEP,” causing lung derecruitment and hypoxemia
-End-expiratory lung volume (EELV) has been used as a surrogate for lung recruitment
Study:
-Small, two center, observational study (46 patients with ARDS)
-50% of patients had a significant increase in EELV after administration of neuromuscular blockade (NMB)
-Statistically significant correlation between a higher dosage of fentanyl and a greater increase in EELV after NMB
Takeaways:
-NMB can improve lung recruitment for a subset of patients with ARDS, particularly in patients with significant expiratory muscle use (this can be seen on your physical exam of your intubated ED boarding patient)
-Although this was not the main point of this study, consider fentanyl-associated “anti-PEEP,” particularly in patients receiving fentanyl whose hypoxemia and/or ventilator mechanics are disproportionate to their imaging
-This can be assessed with NMB (but ensure the patient will have adequate minute ventilation first)
-Naloxone has also been shown to reverse fentanyl-associated rigidity, but obviously would induce patient discomfort/withdrawal
*Of note, because this was an observational trial, it is possible that the patients with increased work of breathing were simply given more fentanyl. Regardless, these findings are consistent with previously documented physiologic side effects of fentanyl.
Plens GM, Droghi MT, Alcala GC, et al. Expiratory muscle activity counteracts positive end-expiratory pressure and is associated with fentanyl dose in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209(5):563-572.
Category: Critical Care
Keywords: IVC, POCUS (PubMed Search)
Posted: 1/17/2024 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
IVC POCUS is often misapplied in attempts to assess volume status and/or volume “responsiveness.” Here are some important concepts to understand when using IVC POCUS to guide management:
Rola P, Haycock K, Spiegel R. What every intensivist should know about the IVC. Journal of Critical Care. Published online November 2023:154455.
Category: Critical Care
Posted: 11/28/2023 by Caleb Chan, MD
(Updated: 2/1/2026)
Click here to contact Caleb Chan, MD
McCallister R, Nuppnau M, Sjoding MW, Dickson RP, Chanderraj R. In patients with sepsis, initial lactate clearance is confounded highly by comorbidities and poorly predicts subsequent lactate trajectory. CHEST. 2023;164(3):667-669.
Category: Critical Care
Posted: 8/15/2023 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Background:
There has been interest in vitamin C as an adjunctive therapy in patients with systemic inflammation and vasoplegia to reduce inflammation. While it was suggested that vitamin C may have some benefit (along with hydrocortisone and thiamine) in septic shock, the LOVIT trial showed possible harm from high-dose vitamin C administration in septic ICU patients. The VALENCIA trial sought to evaluate whether vitamin C could reduce the duration of vasopressor therapy in patients with moderate vasoplegic shock.
Study:
-double-blinded RCT at two tertiary centers, 71 patients (36 to placebo, 35 to vitamin C)
-adult patients with vasoplegic shock of any cause
-vasopressor requirement >10 μg/min of norepi after hypovolemia was excluded
-notable exclusion criteria: end-stage renal failure and expected survival <12 hrs
Results:
-65 pts with septic shock, 6 pts with non-infectious cause
-no significant difference in the duration of vasopressors between the treatment group (median, 44 h [95% CI, 37-54 hrs]) and the control group (55 hrs [95% CI, 33-66 hrs])
-also no statistically significant difference in the vasopressor dose at 12 hourly time points, ICU or 28-day mortality and ICU or hospital length of stay
Take-home points:
Small study that ultimately may be under-powered but did not show that vitamin C reduces vasopressor duration in moderate vasoplegic shock
Anstey MH, Aljeaidi MS, Palmer R, et al. Intravenous vitamin C for vasoplegia: A double-blinded randomised clinical trial (VALENCIA trial). Journal of Critical Care. 2023;78:154369.
Category: Critical Care
Posted: 4/25/2023 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Hypoxemic respiratory failure is a common presentation of critically ill patients. If the degree of hypoxemia is severe and disproportionate to the patient's radiographic findings and not responding to increasing FiO2, a right-to-left shunt should be considered. To evaluate for an anatomic shunt, an intravenous agitated saline contrast (ASC) echocardiographic evaluation can be conducted by an ED provider at the bedside.
Technique:
Interpretation:
Millington SJ, Mayo-Malasky H, Koenig S. Agitated saline contrast injection in patients with severe hypoxemia. J Intensive Care Med. 2023;38(5):479-486.
Category: Critical Care
Posted: 3/1/2023 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Background:
There have been a few studies that suggested that there may be some neuroprotective effect with a higher MAP goal in post-arrest patients. However, these studies were small and/or observational.
Intervention:
-The BOX trial was a double-blind, dual-center (Denmark), randomized trial
-Study population: >18 yo, OHCA of presumed cardiac cause
-Pts randomized to higher (77 mmHg) vs. lower (63 mmHg) MAP goal
-double-blinded by attaching a module that reported a BP that was 10% higher or lower than the pt’s actual BP
-Notable exclusion criteria:
-unwitnessed asystole or suspected intracranial bleeding/stroke
Results/Primary outcome:
-No sig difference in composite of death + Cerebral Performance Category of 3 or 4 (3= severe disability, 4= coma) within 90 days
-133 patients (34%) in the high-target group vs 127 patients (32%) in the low-target group (hazard ratio, 1.08;95%CI, 0.84 to 1.37; P=0.56)
Caveats/Takeaways:
-Mean difference in BP was 10.7 mmHg (95[CI], 10.0 to 11.4) which is still relatively clinically significant, but was lower than their goal difference of 14 mmHg
-They used IVF to target a CVP of 10 mmHg prior to initiation of norepi and used dopamine "if necessary"
-Consider generalizability given study population was patients with presumed cardiac cause of arrest
-Keeping a lower MAP goal of >65 mmHg is reasonable in post-arrest patients
Kjaergaard J, Møller JE, Schmidt H, et al. Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med. 2022;387(16):1456-1466.
Category: Critical Care
Posted: 11/8/2022 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
DOSE VF (DOuble SEquential External Defibrillation for Refractory VF) Trial
Background - High quality data regarding the use of double sequential external defibrillation (DSED) and vector-change (VC) defibrillation in refractory vfib is limited
Study
-Three-group, cluster-randomized, controlled trial in six Canadian paramedic services
-Study population:
-OHCA with refractory vfib (initial presenting rhythm of vfib or pulseless VT that was still present after three consecutive rhythm analyses and standard defibrillations separated by 2 minute intervals of CPR) of presumed cardiac etiology (405 patients)
-Some notable exclusion criteria:
-suspected drug overdose, hypothermia, traumatic cardiac arrest
-Protocol:
-First 3 defib attempts in the standard (anterior-lateral) position
-If remained in vfib after three consecutive shocks randomized to one of:
1. Standard defib for all subsequent attempts (136 pts)
2. VC defib (all subsequent attempts in anterior-posterior position) (144 pts)
3. DSED (applied second set of pads in AP position) with near simultaneously (<1 sec) defib shocks (125 pts)
Results
-Primary outcome: survival to hospital discharge
-38 patients (30.4%) in the DSED group vs. 18 (13.3%) in the standard group (RR 2.21; 95% CI, 1.33 to 3.67) (Fragility index of 9)
-31 patients (21.7%) in the VC group (RR [vs. standard], 1.71; 95% CI, 1.01 to 2.88) (Fragility index of 1)
-Notable secondary outcome: survival with a good neurologic outcome
-34 patients (27.4%) who received DSED vs. 15 patients (11.2%) with standard defibrillation (RR, 2.21; 95% CI, 1.26 to 3.88)
Takeaways/Caveats:
-68% of arrests witnessed, 58% received bystander CPR, median response time of 7.4-7.8 min
-Did not reach planned sample size 2/2 COVID pandemic
-No reporting of post-arrest care (e.g. TTM, PCI)
-Overall rates of survival and good neuro outcome on the higher side even with standard of care
-More/larger studies needed, but can consider DSED for refractory vfib, particularly if you are in a setting without more advanced circulatory support/resources
Cheskes S, Verbeek PR, Drennan IR, et al. Defibrillation strategies for refractory ventricular fibrillation. N Engl J Med. Published online November 6, 2022:NEJMoa2207304.
Category: Critical Care
Posted: 9/13/2022 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Point-of-care ultrasound compression of the carotid artery for pulse determination in cardiopulmonary resuscitation
Background:
S. Y. Kang, I. J. Jo, G. Lee et al., Point-of-care ultrasound compression of the carotid artery for pulse determination in cardiopulmonary resuscitation, Resuscitation, https://doi.org/10.1016/j.resuscitation.2022.06.025
Category: Critical Care
Posted: 7/19/2022 by Caleb Chan, MD
(Updated: 2/1/2026)
Click here to contact Caleb Chan, MD
Tachyarrhythmias in the setting of high-dose vasopressors due to septic shock are not uncommon. Aside from amiodarone, some providers may not know of alternative therapeutic options in the setting of septic shock. In addition, some may view the use of a beta-blocker as counter-intuitive or counter-productive in the setting of norepinephrine usage.
However, there have been multiple smaller studies evaluating using esmolol (and other short-acting beta-blockers) in the setting of tachycardia, septic shock and pressors. Outcomes regarding the theoretical benefits of beta-blockade in sepsis (i.e. decreased mortality/morbidity 2/2 decreased sympathetic innervation, inflammation, myocardial demand etc.) have been varied. However, esmolol has been demonstrated multiple times to be effective at reducing heart rate without significant adverse outcomes (i.e. no sig diff in mortality, refractory shock, or time on vasopressors).
Caveats/pitfalls
-most of the studies discuss “adequate resuscitation” prior to initiation of esmolol
-not studied in patients that also had significant cardiac dysfunction
-be aware that esmolol gtts can be a lot of volume and pts can become volume overloaded if boarding in the ED for an extended period of time
Cocchi MN, Dargin J, Chase M, et al. Esmolol to treat the hemodynamic effects of septic shock: a randomized controlled trial. Shock. 2022;57(4):508-517.
Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683.
Rehberg S, Joannidis M, Whitehouse T, Morelli A. Landiolol for managing atrial fibrillation in intensive care. European Heart Journal Supplements. 2018;20(suppl_A):A15-A18.
Zhang J, Chen C, Liu Y, Yang Y, Yang X, Yang J. Benefits of esmolol in adults with sepsis and septic shock: An updated meta-analysis of randomized controlled trials. Medicine. 2022;101(27):e29820.
Category: Critical Care
Posted: 5/24/2022 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
-If the patient is able to maintain mentation/airway/SpO2/hemodynamics and cough up blood, intubation is not immediately necessary
-If you do intubate, intubate with the largest ETT possibly to faciliate bronchoscopic interventions and clearance of blood
-The CT scan that typically needs to be ordered is a CTA (not CTPA) with IV con
-See if you can find prior/recent imaging in the immediate setting (e.g. pre-existing mass/cavitation on R/L/upper/lower lobes)
-Get these meds ready before the bronchoscopist gets to the bedside to expedite care:
-If the pt's vent suddenly has new high peak pressures or decreased volumes after placement of endobronchial blocker, be concerned that the blocker has migrated
Charya AV, Holden VK, Pickering EM. Management of life-threatening hemoptysis in the ICU. J Thorac Dis. 2021;13(8):5139-5158.
Category: Critical Care
Posted: 12/7/2021 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Clinical Pearls for Variceal Hemorrhage
-lower mortality with “restrictive” (Hgb 7-9 g/dL) rather than liberal strategy
-antibiotic “prophylaxis” reduces mortality
-no need to correct INR with FFP
-vasoactives (i.e. octreotide, somatostatin, terlipressin) alone may actually control bleeding
-for your ICU boarders...if persistent or severe rebleeding (despite endoscopic therapy), rescue TIPS is therapy of choice (call IR)
Zanetto A, Shalaby S, Feltracco P, et al. Recent advances in the management of acute variceal hemorrhage. Journal of Clinical Medicine. 2021;10(17):3818.
Category: Critical Care
Posted: 3/2/2021 by Caleb Chan, MD
(Updated: 2/1/2026)
Click here to contact Caleb Chan, MD
Clinical Question:
Methods:
Results:
Take-home points:
Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. Published online February 2, 2021:NEJMoa2024922.
Category: Critical Care
Posted: 1/6/2021 by Caleb Chan, MD
Click here to contact Caleb Chan, MD
Study Question: What is the association of relative hypotension (degree and duration of MPP deficit) in patients with vasopressor-dependent shock with the incidence of new significant AKI and major adverse kidney events (MAKE)?
Methods:
Results:
Take-aways:
Panwar R, Tarvade S, Lanyon N, et al. Relative hypotension and adverse kidney-related outcomes among critically ill patients with shock. A multicenter, prospective cohort study. Am J Respir Crit Care Med. 2020;202(10):1407-1418.
