Category: Toxicology
Keywords: ketamine, agitation, prehospital, haloperidol (PubMed Search)
Posted: 6/7/2016 by Bryan Hayes, PharmD
(Updated: 6/27/2016)
Click here to contact Bryan Hayes, PharmD
Ketamine is gaining traction as a prehospital option for managing severe agitation or excited delirium syndrome. Previous reports have mostly been case series, but a new prospective study adds some important information that may help delineate ketamine's role in this setting. [1] The study and an accompanying commentary are both open access. [2]
What They Did
Open-label before-and-after prospective comparison of haloperidol (10 mg IM) versus ketamine (5 mg/kg IM) for the treatment of acute undifferentiated agitation.
What They Found
Appliation to Clinical Practice
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: clindamycin, trimethoprim-sulfamethoxazole, wound infection, TMP-SMX (PubMed Search)
Posted: 6/2/2016 by Bryan Hayes, PharmD
(Updated: 6/4/2016)
Click here to contact Bryan Hayes, PharmD
In settings where community-acquired MRSA is prevalent, which antibiotic is best for uncomplicated wound infections?
New Study
What They Found
Application to Clinical Practice
Talan DA, et al. A randomized trial of clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated wound infection. Clin Infect Dis 2016;62(12):1505-13. [PMID 27025829]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: digoxin, chronic, poisoning, immune Fab (PubMed Search)
Posted: 5/9/2016 by Bryan Hayes, PharmD
(Updated: 5/12/2016)
Click here to contact Bryan Hayes, PharmD
Patients with chronic digoxin toxicity generally have multiple co-morbidities such as renal failure, dehydration, and cardiac failure. Sick patients with chronically high digoxin levels may have more than just digoxin toxicity as the cause of illness.
A New Study
Prospective observational study with the primary objective to investigate changes in free digoxin concentrations and clinical effects on heart rate and potassium concentrations in chronic digoxin poisoning when digoxin immune Fab are given.
What They Found
One to two vials of digoxin immune Fab initially bound all free digoxin confirming Fab efficacy. However, this was associated with only a moderate improvement in HR (49 to 57 bpm) and potassium (5.3 to 5.0 mmol/L).
Application to Clinical Practice
Chan BS, et al. Efficacy and effectiveness of anti-digoxin antibodies in chronic digoxin poisonings from the DORA study (ATOM-1). Clin Toxicol. 2016 Apr 27. Epub ahead of print. [PMID 27118413]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: ketamine, shock index, hemodynamic, prehospital, RSI (PubMed Search)
Posted: 5/3/2016 by Bryan Hayes, PharmD
(Updated: 5/7/2016)
Click here to contact Bryan Hayes, PharmD
Ketamine is often thought to be the induction agent least associated with hypotension in the peri-intubation period. However, reports of hypotension following ketamine do exist, including 2 cases of cardiac arrest. [1] There are limited objective means to predict which patients may have an adverse hemodynamic response.
New Study
A new prospective observational study followed 112 patients in the prehospital setting who received ketamine for rapid sequence intubation. 81 had a low shock index [< 0.9], 31 had a high shock index. [2]
Shock index = HR / SBP
What They Found
Patients with a high shock index were more likely to experience hypotension (SBP < 90 mm Hg) in the peri-intubation period compared to those with a low shock index (26% vs 2%).
Application to Clinical Practice
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: Extracorporeal Membrane Oxygenation, ECMO, toxicology, poison (PubMed Search)
Posted: 4/13/2016 by Bryan Hayes, PharmD
(Updated: 4/14/2016)
Click here to contact Bryan Hayes, PharmD
The American College of Medical Toxicology's ToxIC Registry is a self-reporting database completed by medical toxicologists across 69 insitutions in the US.
Application to Clinical Practice
In settings where ECMO is available, it may be a potential treatment option in severely poisoned patients. From the limited data, ECMO was generally administered prior to cardiovascular failure and might be of benefit particularly during the time the drug is being metabolized.
Table from the Case Series
Wang GS, et al. Extracorporeal Membrane Oxygenation (ECMO) for Severe Toxicological Exposures: Review of the Toxicology Investigators Consortium (ToxIC). J Med Toxicol 2016;12(1):95-9. [PMID 26013746]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: vancomycin, loading dose, nephrotoxicity (PubMed Search)
Posted: 3/24/2016 by Bryan Hayes, PharmD
(Updated: 4/2/2016)
Click here to contact Bryan Hayes, PharmD
Guidelines recommend loading doses of vancomycin (15-20 mg/kg, up to 30 mg/kg in critically ill patients), but the risk of nephrotoxicity is unknown. A new retrospective cohort study aimed to compare nephrotoxicity in ED sepsis patients who received vancomycin at high doses (>20 mg/kg) versus lower doses (20 mg/kg).
What They Found
1,330 patients had three SCr values assessed for the primary outcome
High-dose initial vancomycin was actually associated with a lower rate of nephrotoxicity (5.8% vs 11.1%)
After adjusting for age, gender, and initial SCr, the risk of high dose vancomycin compared to low dose was decreased for the development of nephrotoxicity (RR=0.60; 95% CI: 0.44, 0.82)
Application to Clinical Practice
It appears initial loading doses of vancomcyin > 20 mg/kg do not cause increased risk of nephrotoxicity.
Rosini JM, et al. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad Emerg Med. 2016 Feb 6. Epub ahead of print. [PMID 26850378]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: cocaine, toxicity, cardiovascular (PubMed Search)
Posted: 3/9/2016 by Bryan Hayes, PharmD
(Updated: 3/12/2016)
Click here to contact Bryan Hayes, PharmD
Acute cocaine toxicity can manifest with several cardiovascular issues such as tachycardia, dysrhythmia, hypertension, and coronary vasospasm. A new systematic review collated all of the available evidence for potential treatment options. Here is what the review found (there is also an 'other agents' section for medications with less published reports):
Benzodiazepines and other GABA-active agents: Benzodiazepines may not always effectively mitigate tachycardia, hypertension, and vasospasm from cocaine toxicity.
Calcium channel blockers: Calcium channel blockers may decrease hypertension and coronary vasospasm, but not necessarily tachycardia.
Nitric oxide-mediated vasodilators: Nitroglycerin may lead to severe hypotension and reflex tachycardia.
Alpha-adrenoceptor blocking drugs: Alpha-1 blockers may improve hypertension and vasospasm, but not tachycardia, although evidence is limited.
Alpha-2-adrenoceptor agonists: There were two high-quality studies and one case report detailing the successful use of dexmedetomidine.
Beta-blockers and alpha/beta-blockers: No adverse events were reported for use of combined alpha/beta-blockers such as labetalol and carvedilol, which were effective in attenuating both hypertension and tachycardia.
Antipsychotics: Antipsychotics may improve agitation and psychosis, but with inconsistent reduction in tachycardia and hypertension and risk of extrapyramidal adverse effects.
Sodium bicarbonate: Twelve case reports documented treatment of dysrhythmia with IV sodium bicarbonate, with seven reporting successful termination.
The authors note that "publication bias is a concern, and it is possible that successful treatment and/or adverse events have not been reported in some of the publications, and in general."
Richard JR, et al. Treatment of cocaine cardiovascular toxicity: a systematic review. Clin Toxicol. 2016 Feb 26:1-20. [Epub ahead of print] [PMID 26919414]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: status epilepticus (PubMed Search)
Posted: 3/3/2016 by Bryan Hayes, PharmD
(Updated: 3/5/2016)
Click here to contact Bryan Hayes, PharmD
A new guideline for convulsive status epilepticus in adults AND children was recently published. [1] An insightful commentary was published alongside it (both are open access). [2] The proposed algorithm is below. Here are a few additional points to note:
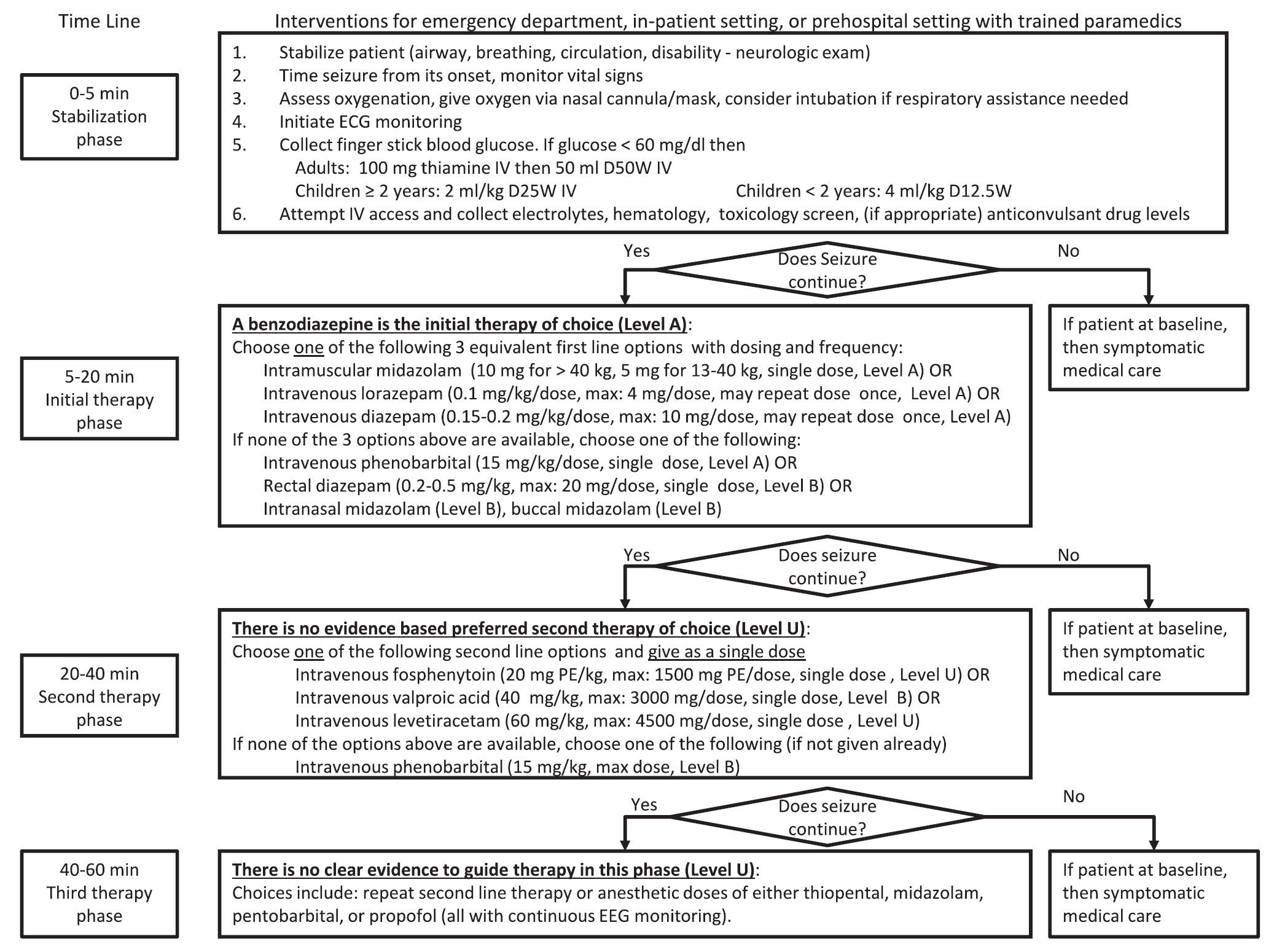
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: lipid, intralipid, poisoning, local anesthetic, non-local anesthetic (PubMed Search)
Posted: 2/10/2016 by Bryan Hayes, PharmD
(Updated: 4/2/2016)
Click here to contact Bryan Hayes, PharmD
In September 2013, an international group representing major societies in toxicology and nutrition support began collaborating on a comprehensive review of lipid use in poisoning. Six total papers will be published, with the most recent two made available online this week. Here are the available (and forthcoming) papers:
Gosselin S, et al. Methodology for AACT evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol 2015;53(6):557-64. [PMID 26059735]
Grunbaum AM, et al. Review of the effect of intravenous lipid emulsion on laboratory analyses. Clin Toxicol 2016:54(2):92-102. [PMID 26623668]
Levine M, et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol. 2016;54(3):194-221. [PMID 26852931]
Hoegberg LC, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol. 2016;54(3):167-93. [PMID 26853119]
Hayes BD, et al. Systematic Review of Clinical Adverse Events Reported After Acute Intravenous Lipid Emulsion Administration. Clin Toxicol. 2016 Apr 1. [Epub ahead of print] [PMID 27035513]
The final paper, which is in process, is the consensus recommendations from the workgroup based on the 4 systematic reviews.
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: succinylcholine, rocuronium, mortality, traumatic brain injury, RSI (PubMed Search)
Posted: 2/4/2016 by Bryan Hayes, PharmD
(Updated: 2/6/2016)
Click here to contact Bryan Hayes, PharmD
An interesting new study was published looking at in-hospital mortality in TBI patients who received succinylcholine or rocuronium for RSI in the ED.
What They Did
What They Found
Application to Clinical Practice
Patanwala AE, et al. Succinylcholine is associated with increased mortality when used for rapid sequence intubation of severely brain injured patients in the emergency department. Pharmacotherapy 2016;36(1):57-63. [PMID 26799349]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: acetaminophen, acetylcysteine (PubMed Search)
Posted: 1/7/2016 by Bryan Hayes, PharmD
(Updated: 1/14/2016)
Click here to contact Bryan Hayes, PharmD
The three-bag IV acetylcysteine regimen for acetaminophen overdose is complicated and can result in medication/administration errors. [1] Two recent studies have attempted simplifying the regimen using a two-bag approach and evaluated its effect on adverse effects. [2, 3]
Study 1 [2]
Prospective comparison of cases using a 20 h, two-bag regimen (200 mg/kg over 4 h followed by 100 mg/kg over 16 h) to an historical cohort treated with the 21 h three-bag IV regimen (150 mg/kg over 1 h, 50 mg/kg over 4 h and 100 mg/kg over 16 h).
The two-bag 20 h acetylcysteine regimen was well tolerated and resulted in significantly fewer and milder non-allergic anaphylactic reactions than the standard three-bag regimen.
Study 2 [3]
Prospective observational study of a modified 2-phase acetylcysteine protocol. The first infusion was 200 mg/kg over 4-9 h. The second infusion was 100 mg/kg over 16 h. Pre-defined outcomes were frequency of adverse reactions (systemic hypersensitivity reactions or gastrointestinal); proportion with ALT > 1000 U/L or abnormal ALT.
The 2-phase acetylcysteine infusion protocol resulted in fewer reactions in patients with toxic paracetamol concentrations.
Final word: Two-bag regimens seem to offer advantages compared to the traditional three-bag regimen with regard to reduced adverse drug reactions. Look for more data, particularly on effectiveness, and a potential transition to a two-bag approach in the future.
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: sugammadex, rocuronium, NMBA, vecuronium (PubMed Search)
Posted: 12/29/2015 by Bryan Hayes, PharmD
(Updated: 1/2/2016)
Click here to contact Bryan Hayes, PharmD
After three failed attempts, the FDA finally granted approval for Merck's non-depolarizing neuromuscular blocker reversal agent sugammadex (Bridion). Though the product has been used in Europe and Asia for several years, hypersensitivity concerns led to the delayed approval in the U.S.
Important points
Application to Clinical Practice
The EM PharmD blog discusses sugammadex's approval in more detail.
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: laboratory, lipid, toxicology (PubMed Search)
Posted: 12/10/2015 by Bryan Hayes, PharmD
Click here to contact Bryan Hayes, PharmD
The American Academy of Clinical Toxicology's Lipid Emulsion workgroup has published its first of 4 systematic reviews on the use of lipid emulsion in toxicology, this one on lipid's effect on laboratory analyses. [1] As expected, administering a fat bolus can significantly alter labs drawn subsequently.
The key point: If you are considering lipid for overdose, draw labs prior to giving it.
Which labs are affected? Most. Here's a helpful mnemonic courtesy of Dr. Kyle DeWitt.
Also remember to give lipid in its own line. It isn't compatable with most resuscitation drugs. [2]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: tramadol, seizure (PubMed Search)
Posted: 12/3/2015 by Bryan Hayes, PharmD
(Updated: 7/6/2016)
Click here to contact Bryan Hayes, PharmD
Tramadol has a reputation for being a safe, non-opioid alternative to opioids. Nothing could be further from the truth. Several blogs have published about the dangers of tramadol:
But what about seizure risk? Previous studies have been unable to confirm an increased seizure risk with therapeutic doses of tramadol (Seizure Risk Associated with Tramadol Use from EM PharmD blog). However, a new study refutes that premise.
22% of first-seizure patients had recent tramadol use!
This was a retrospecitve study without laboratory confirmation of tramadol intake. Nevertheless, it behooves us not to think of tramadol as a safer alternative to opioids. It is an opioid after all, and it comes with significant adverse effects.
Asadi P, et al. Prevalence of Tramadol Consumption in First Seizure patients; a One-Year Cross-sectional Study. Emerg (Tehran) 2015;3(4):159-61. [PMID 26495407]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: Andexanet, apixaban, rivaroxaban, factor Xa (PubMed Search)
Posted: 11/12/2015 by Bryan Hayes, PharmD
Click here to contact Bryan Hayes, PharmD
Not to be outdone by the recent FDA approval of Idarucizumab to reverse dabigatran, a new factor Xa reversal agent is under investigation. "Andexanet binds and sequesters factor Xa inhibitors within the vascular space, thereby restoring the activity of endogenous factor Xa and reducing levels of anticoagulant activity, as assessed by measurement of thrombin generation and anti factor Xa activity, the latter of which is a direct measure of the anticoagulant activity."
Design
Two parallel randomized, placebo-controlled trials (ANNEXA-A [apixaban] and ANNEXA-R [rivaroxaban]) were conducted in healthy vounteers to evaluate the ability of andexanet to reverse anticoagulation, as measured by the percent change in anti factor Xa activity after administration.
What they Found
Compared to placebo, andexanet significantly reduced anti-factor Xa activity, increased thrombin generation, and decreased unbound drug concentration in both the apixaban and rivaroxaban groups.
Application to Clinical Practice
Siegal DM, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. November 11, 2015.
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: low back pain, opioids, naproxen, oxycodone, cyclobenzaprine (PubMed Search)
Posted: 10/21/2015 by Bryan Hayes, PharmD
(Updated: 11/7/2015)
Click here to contact Bryan Hayes, PharmD
If there weren't enough reasons to avoid opioids, here is another: opioids don't work for low back pain (LBP).
Objective
A well-done, double-blind, randomized controlled trial from JAMA set out to compare functional outcomes and pain at 1 week and 3 months after an ED visit for acute LBP among patients randomized to a 10-day course of (1) naproxen + placebo; (2) naproxen + cyclobenzaprine; or (3) naproxen + oxycodone/acetaminophen.
Intervention
Outcome
Neither oxycodone/acetaminophen nor cyclobenzaprine improved pain or functional outcomes at 1 week compared to placebo, and more adverse effects were noted.
Application to Clinical Practice
Among patients with acute, nontraumatic, nonradicular LBP presenting to the ED, avoid adding opioids or cyclobenzaprine to the standard NSAID therapy.
Friedman BW, et al. Naproxen with Cyclobenzaprine, Oxycodone/Aceaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA 2015;314(15):1572-80.
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: hemodialysis, dabigatran, rebound (PubMed Search)
Posted: 10/7/2015 by Bryan Hayes, PharmD
(Updated: 10/8/2015)
Click here to contact Bryan Hayes, PharmD
In patients receiving renal replacement therapy as a treatment modality for dabigatran-related bleeding, watch for a rebound concentration increase after hemodialysis is stopped.
More than 50% of patients demonstrate a rebound effect with a median increase in dabigatran concentration of 33%.
It is unclear whether this rebound effect is clinically important, and whether it translates to prolonged clinically relevant bleeding. Extended hemodialysis sessions or consideration of CVVHD should offset this potential problem.
Bonus Pearl:
The North American Congress of Clinical Toxicology starts today and runs through October 12. Look for toxicology pearls and updates on Twitter under the official conference hashtag #NACCT15.
Chai- Adisaksopha C, et al. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost 2015;13(10):1790-8. [PMID 26270886]
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: targeted temperature management, drug (PubMed Search)
Posted: 9/27/2015 by Bryan Hayes, PharmD
(Updated: 10/3/2015)
Click here to contact Bryan Hayes, PharmD
An excellent new review article provides a detailed look at how the drugs we give are affected by targeted temperature management. Here is a helpful chart of drug alterations that have data in reduced body temperature states:
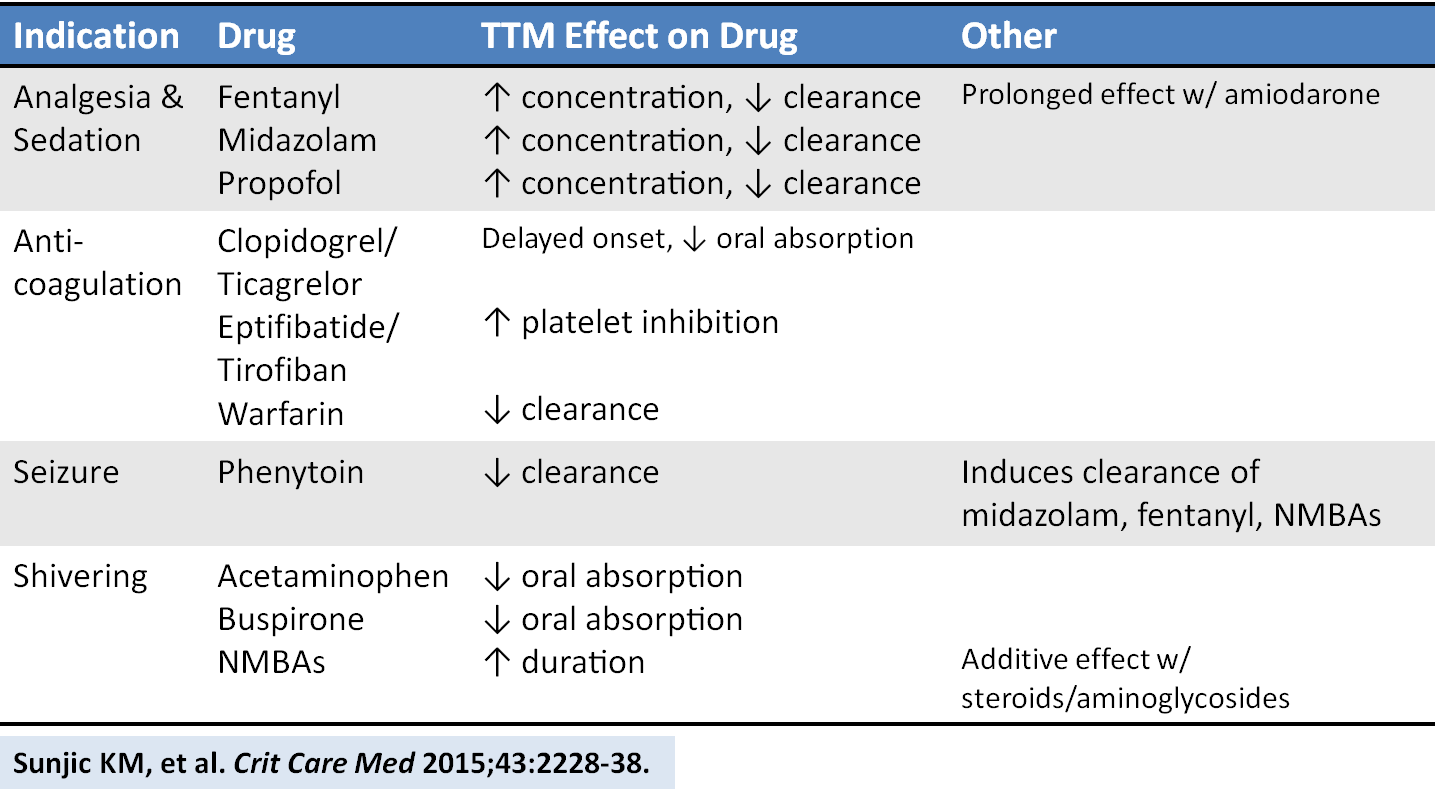
Sunjic KM, et al. Pharmacokinetic and other considerations for drug therapy during targeted temperature management. Crit Care Med 2015;43:2228-38. [PMID 26237312]
Follow me on Twitter (@PharmERToxGuy)
Category: Toxicology
Keywords: eye drops, pupil size, ophthalmic (PubMed Search)
Posted: 9/8/2015 by Bryan Hayes, PharmD
(Updated: 9/11/2015)
Click here to contact Bryan Hayes, PharmD
In the evaluation of ED patients, it may be important to understand the effect on pupil size from the ophthalmic medications they use. Here is a summary chart of common eye drops and their effect on pupil size.
Follow me on Twitter (@PharmERToxGuy)
Category: Pharmacology & Therapeutics
Keywords: ketamine, analgesia, morphine, pain (PubMed Search)
Posted: 8/30/2015 by Bryan Hayes, PharmD
(Updated: 9/5/2015)
Click here to contact Bryan Hayes, PharmD
A new prospective, randomized, double-blind trial compared subdissociative ketamine to morphine for acute pain in the ED.
What they did
What they found
Motov S, et al. Intravenous subdissociative-dose ketamine versus morphine for analgesia in the emergency department: a randomized controlled trial. Ann Emerg Med 2015;66:222-9. [PMID 25817884]
Follow me on Twitter (@PharmERToxGuy)
