Category: Pharmacology & Therapeutics
Posted: 12/9/2025 by Ashley Martinelli
(Updated: 12/11/2025)
Click here to contact Ashley Martinelli
Sympathetic crashing acute pulmonary edema (SCAPE) is an acute, aggressive pulmonary edema that occurs in patients with hypertensive emergencies. Nitroglycerin (NTG) is often utilized in combination with non-invasive positive pressure ventilation to prevent decompensation; however, data is lacking regarding the optimal dosing strategy.
Study design: retrospective, single-center, cohort study at an academic medical center
Inclusion: adult patients with a primary or secondary diagnosis of pulmonary edema, acute heart failure exacerbation, hypertensive emergency, or hypertensive crisis and were initiated on NTG in the ED.
Exclusion: hypertensive emergency with different BP goals (dissection, eclampsia, ICH)
Study groups: based on initial NTG dose (<100 mcg/min = low dose, ? 100 mcg/min = high dose)
Primary outcome: time from NTG initiation to oxygen weaning (removal of necessary oxygen back to baseline or home oxygen
Baseline: 61 years old, 50% male, 97% with history of hypertension, 84% history of heart failure, and 36% with ESRD. A higher percentage of patients in the high dose group has CPAP/BIPAP (49% vs 27% p<0.001)
Results: High dose NTG group had a shorter time from NTG to oxygen wean of 2.67h compared to 3.28 hours in the low group. The high dose group also was more likely to achieve goal SBP reduction of 25% within the hour (55% v 34%, p<0.001) had a shorter duration of NTG infusion overall 4.9h vs 6.9h, p0.033) and had decreased ICU LOS by 0.5 days. There were more cases of hypotension in the high dose group which was primarily driven by acute drops in SBP >30%.
Bottom Line: Consider using NTG 100 mcg/min initially to manage patients with SCAPE in the ED.
Henry K, Pelsue B, Hartman H, Gulbis B. Low versus high dosing strategies of intravenous nitroglycerin for the management of sympathetic crashing acute pulmonary edema. Am J Emerg Med. 2025; 98:41-45.
Category: Pharmacology & Therapeutics
Keywords: steroids, asthma, copd (PubMed Search)
Posted: 8/7/2025 by Ashley Martinelli
(Updated: 8/14/2025)
Click here to contact Ashley Martinelli
There are various reasons to give corticosteroids in the emergency department. Many decisions regarding IV vs PO, and the numerous available products can lead to excessive dosing (such as 125mg methylprednisolone). Below is a reference for the most common indications as well as conversion recommendations for each product
Guideline Recommended Dosing for Common ED Indications:
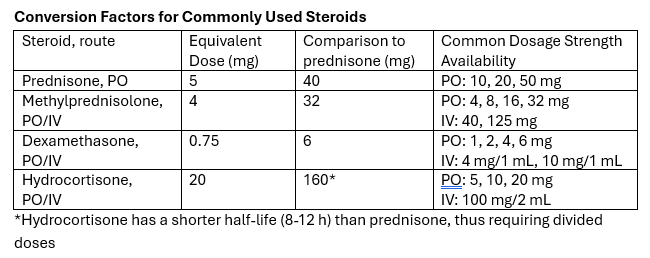
Take-away: Methylprednisolone 125mg is frequently requested but provides a dose equivalent to prednisone 150mg. Consider guideline directed dosing and conversion of products to prevent excessive initial steroid dosing.
Category: Pharmacology & Therapeutics
Keywords: tenecteplase, alteplase, stroke (PubMed Search)
Posted: 3/10/2025 by Ashley Martinelli
(Updated: 3/13/2025)
Click here to contact Ashley Martinelli
On March 3, 2025, the FDA approved tenecteplase to treat acute ischemic stroke. Historically, only alteplase was FDA-approved, but the stroke guidelines suggest tenecteplase as a reasonable alternative and many centers have made the change to use tenecteplase.
The EXTEND-IA TNK trial showed benefit of tenecteplase over alteplase in patients who were candidates for mechanical thrombectomy. The newer AcT trial found that tenecteplase was non-inferior to alteplase for patients eligible for thrombolysis, regardless of thrombectomy candidacy. There was no difference in safety outcomes, specifically ICH or angioedema in either trial.
Tenecteplase will soon be available in a new 25 mg vial with stroke-specific packaging (potentially as early as June 2025). Currently, there is only a 50 mg vial that is used for STEMI and PE which has higher maximum dosing compared to stroke.
The dosing is now recommended in weight-based groupings based on the supplemental appendix from the AcT trial. This is likely a change in practice for most centers that previously implemented tenecteplase for stroke before the FDA approval. Consult with your stroke and pharmacy team to discuss potential protocol changes at your institution.

Campbell BCV, et al. NEJM 2018;378:1573-1582.
Menon BK, et al. Lancet 2022;400:161-69.
TNKase [package insert]. South San Francisco, CA. Genetech, Inc. 2025.
Genetech Press Release: https://www.gene.com/media/press-releases/15053/2025-03-03/fda-approves-genentechs-tnkase-in-acute-#:~:text=South%20San%20Francisco%2C%20CA%20%2D%2D,stroke%20(AIS)%20in%20adults.
Category: Trauma
Keywords: ketamine, trauma (PubMed Search)
Posted: 11/14/2024 by Ashley Martinelli
Click here to contact Ashley Martinelli
An out-of-hospital, randomized, placebo-controlled, blinded, parallel group study was conducted in adult patients under the care of the city fire-based emergency medical services and the local level one trauma center. Adult male patients experiencing moderate to severe pain due to traumatic injuries received either 50mg of intranasal ketamine or placebo in addition to fentanyl after randomization in the field by the paramedic (a novel approach). The primary outcome was reduction of pain by 2 points 30 minutes after study drug administration.
199 patients were randomized with 107 receiving ketamine and 92 with placebo. Patients were young (30-40), and had a median weight of 83 kg. Pretreatment pain scores were 10/10 and patients presented to the ED 14 minutes after receiving study medication. The most common injuries were falls, MVC, and GSW. Half of the patients received IV fentanyl but others had IM or IN routes.
Ketamine receipt did not lead to a 2 point reduction in pain scores (36% vs 44.7% p = 0.22). There was no difference in pain at 3 hours, additional medications received, or total amount of analgesia received. Notably, there were no differences in adverse events.
McMullan JT, Droege CA, Chard KM, Otten EJ, Hart KW, Lindsell CJ, Strilka RJ. Out-of-Hospital Intranasal Ketamine as an Adjunct to Fentanyl for the Treatment of Acute Traumatic Pain: A Randomized Clinical Trial. Ann Emerg Med. 2024 Oct;84(4):363-373. doi: 10.1016/j.annemergmed.2024.04.018. Epub 2024 Jun 12. PMID: 38864781.
Category: Obstetrics & Gynecology
Keywords: breastfeeding, lactation (PubMed Search)
Posted: 8/7/2024 by Ashley Martinelli
(Updated: 8/8/2024)
Click here to contact Ashley Martinelli
Breastfeeding provides a great nutrition source for infants, but early cessation is common for a wide variety of reasons. Notably, being asked to withhold breastmilk (“pump and dump”) due to safety concerns or illness increases rates of termination.
A recent paper is an invaluable reference on commonly used medications in the care of emergency department women of childbearing age and the lactation risk. It breaks down medications into clinical categories and then further highlights those that are safe, likely safe, and safe-but may reduce milk supply, and those to avoid.
The majority of commonly used medications in the ED are safe to use in breastfeeding. Only 3% of the medication analyzed should be avoided (aspirin [at doses > 325mg/day], dicyclomine, prochlorperazine, and benzonatate) and in most cases a safe alternative could be used.
Using these recommendations can help prescribe safe medications, prevent the recommendations to pump and dump, and relieve stress for the patient breastfeeding.
Consider adding the LactMed(R) app to your phone as well - This is a free database through the NIH to search individual medications to assess risk in lactation.
Premer C, Caruso K. Safety profile of the most ordered medications for breastfeeding patients in the emergency department. Am J Emerg Med. 2024;80:1-7.
Category: Pharmacology & Therapeutics
Keywords: naloxone, opioid (PubMed Search)
Posted: 4/11/2024 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Naloxone is given frequently in the emergency department to improve the respiratory rate in patients with suspected or known opioid ingestion. In order to minimize the risk of severe opioid withdrawal (nausea, vomiting, diarrhea, anxiety, piloerection, sweating, agitation, etc.), consider diluting naloxone and administering small aliquots of 0.04-0.08mg at a time. This requires IV access and a patient with a present, but low respiratory rate.
Dilution instructions:
Supplies:
Instructions:
Administer 1 -2 mL (0.04 – 0.08 mg) naloxone every 2 minutes and assess response.
Don't forget to prescribe/give naloxone upon discharge from the emergency department.
Category: Pharmacology & Therapeutics
Posted: 12/14/2023 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Bottom Line: Droperidol is an effective alternative to haloperidol in the treatment of gastroparesis although most patients will also receive prokinetic agents as well such as metoclopramide. It may also have some analgesic benefit.
Prior studies have demonstrated the efficacy and safety of haloperidol in the management of gastroparesis. A recent retrospective study was conducted to assess the impact of droperidol as it is an effective antiemetic similar to haloperidol.
This study enrolled 233 patients. Visits were matched with their most recent ED visit > 7 prior where droperidol was not administered.
Most patients were female, 51% African American, and the median age was 40. Doses ranged from 0.625 mg – 2.5 mg with the most common dose being 1.25 mg.
Results:
Stirrup N, Jones G, Arthus J, Lewis Z. Droperidol undermining gastroparesis symptoms (DRUGS) in the emergency department. American Journal of Emergency Medicine. 2024;75:42-45.
Category: Pharmacology & Therapeutics
Keywords: contraception (PubMed Search)
Posted: 5/11/2023 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Emergency contraception is highly effective at preventing unwanted pregnancies and has been on the market for 20+ years.
Levonogestrel (LNG) 1.5 mg PO x 1 dose (OTC Available)
Ulipristal acetate (UPA) 30 mg PO x 1 dose (Requires RX)
Original studies with LNG was estimated to prevent up to 80% of expected pregnancies. In the subsequent trials that brought UPA to the market and compared the two medications, LNG prevented 69% (95% CI, 46-82%) and 52.2% (95% CI, 25.1-69.5%).
While pregnancy rates are low with both options there is concern with patients of higher weight/BMI that the effectiveness of levonorgestrel decreases as weight rises. One large study of over 1700 patients specifically noted that a weight > 75 kg was associated with up to 6.5% pregnancy rate (95% CI 3.1-11.5) compared to 1.4% (95% CI 0.5-3.0) in patients weighing 65-75 kg. Patients weighing > 85 kg had similarly high rates at 5.7% (95% CI 2.9-10.0).
The cost difference is minimal between products, especially when considering costs associated with treatment failures and subsequent need for care- the largest difference is with respect to access as LNG is OTC and UPA requires an RX. Either can be administered in an ED setting as long as they are on formulary.
ACOG also recommends that ulipristal be utilized for it higher overall efficacy compared to levonorgestrel.
Consider:
For patients above 75 kg, ulipristal can be used as first line emergency contraception for up to 5 days following unprotected intercourse.
Patients < 75 kg and < 72 hours following unprotected intercourse can use levonorgestrel or ulipristal as an appropriate emergency contraception method.
Patients < 75 kg and 72-120 hours following unprotected intercourse should use ulipristal due to its efficacy beyond 72 hours.
Kapp N, Abitbol JL, Mathe H, et al. Effect of body weight and BMI on the efficacy of levonogestrel emergency contraception. Contraception. 2015;91(2):97-104.
Emergency contraception. Practice Bulletin No. 152. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e1–11
Category: Pharmacology & Therapeutics
Keywords: atrial fibrillation, atrial flutter, diltiazem, calcium (PubMed Search)
Posted: 3/3/2023 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Non-dihydropyridine calcium channel blockers, verapamil and diltiazem, can induce hypotension when administered intravenously (IV) in approximately 4% of patients. It has previously been taught that administering IV calcium before administering these medications may prevent the hypotension. Previously, this theory was tested for verapamil and found success with reducing hypotension. Only one study has been done exclusively with diltiazem and it found no benefit.
In a new multicenter retrospective cohort study of adults in the ED, patients were randomized into two groups: those who received diltiazem alone and those who received calcium with diltiazem for atrial fibrillation/atrial flutter (AF/AFL) with a HR ≥ 120 bpm. Patients were excluded if they required electrocardioversion, had other agents prior to diltiazem, incomplete information, were pregnant or incarcerated. The primary outcome was change in SBP 60 minutes (+/-30 minutes) after diltiazem administration.
Baseline characteristics: 73 year old, equal male:female, predominantly white patients. 40% had new onset AF/AFL and the initial HR was 140 in both groups. There were 198 patients in the diltiazem group and 56 patients in the combination group. Notably, patients in the combination group had a lower presenting SBP 109 (101-121) vs 123 (114-132) P<0.0001 which matches classical teaching for when to consider calcium use. Additionally, patients in the combination group received a lower diltiazem dose of 10mg vs 15mg in the monotherapy group p=0.004 with both group receiving doses lower than the standard 0.25 mg/kg dosing recommendation.
Outcomes:
Take Home Point:
Administration of IV calcium may not be as beneficial as previously thought to prevent hypotension induced by diltiazem administration. This particular study is confounded by the relatively low doses of diltiazem overall, but utilizing a lower dosing strategy in patients with low SBP is a reasonable safety strategy.
Rossi N, Allen B, Hailu K, et al. Impact of intravenous calcium with diltiazem for atrial fibrillation/flutter in the emergency department. Am J Emergency Medicine. 2023;64:57-61.
Category: Pharmacology & Therapeutics
Keywords: sepsis, piperacillin-tazobactam (PubMed Search)
Posted: 9/29/2022 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Piperacillin-tazobactam is one of the most commonly used antipseudomonal antibiotics in the empiric management of patients with septic shock. The package insert recommends dose reductions for renal impairment in other infectious etiologies, but the impact of dose reduction has not been previously studied in patients with septic shock.
A recent retrospective, observational cohort study compared outcomes of patients with septic shock who received ≥ 27 grams (at least 3.375 gm q6 hours x 48 h-“NORM”) versus those who received < 27 grams (“LOW”) over the initial 48 h of septic shock (defined as concomitant norepinephrine infusion).
Patients were excluded if they had death or hospice disposition within the 48h study period. The primary outcome was the number of norepinephrine free days (NFD) at day 28. Propensity matching was utilized to account for confounders.
Results: 351 in the LOW group, 928 in the NORM group with 608 pairs in the propensity matched assessment.
Bottom Line: Dose reductions of piperacillin-tazobactam appears to be harmful early in the management of patients with septic shock.
JM Allen, Surajbali D, Ngyuen DQ, et al. Impact of piperacillin-tazobactam dosing in septic shock patients using real-world evidence: an observational retrospective cohort study. Ann Pharmacotherapy; 2022: Sep 25:10600280221125919. doi: 10.1177/10600280221125919. Epub ahead of print. PMID: 36154486.
Category: Pharmacology & Therapeutics
Keywords: insulin, hyperkalemia, hypoglycemia (PubMed Search)
Posted: 7/4/2022 by Ashley Martinelli
Click here to contact Ashley Martinelli
Prior studies have found that patients are at an increased risk for hypoglycemia when administered insulin for the acute management of hyperkalemia when they have renal dysfunction. A new single-center, retrospective study investigated the risk of hypoglycemia and the overall effect of potassium lowering in patients with renal dysfunction and stratified outcomes based on the CKD level.
Patients were included if they were ordered insulin for hyperkalemia using a hospital driven order set and had CKD stages 3a, 3b, and 4. They were excluded if they had dialysis within 6h of insulin administration, had DKA, or no repeat labs. The hospital order set encourages 5 units of insulin instead of 10 when “renal failure” is present without clear guidance.
377 patients were included: 186 received 5 units and 191 received 10 units. The average age was 65 years old, predominantly male, weighing 90 kg. In the 5 unit group, significantly more patients had CKD stage 4 (60% v 30%) and in the 10 unit group, significantly more patients were CKD stage 3a (p<0.001). The baseline serum potassium was 6 in each group.
The hypoglycemia incidence was not different between groups, with severe hypoglycemia occurring twice per group. All patients received dextrose according to the protocol.
There was a significant difference in the reduction of serum potassium between the 5 and 10 unit groups: -0.63 mmol/L vs -0.9 mmol/L (p 0.001).
Bottom line: Hypoglycemia occurred even with insulin dose reduction. Potassium lowering was higher in patients who received the 10 unit dose.
Finder SN, McLaughlin LB, Dillon RC. 5 versus 10 units of intravenous insulin for hyperkalemia in patients with moderate renal dysfunction.
Category: Pharmacology & Therapeutics
Keywords: haloperidol, agitation, sedation (PubMed Search)
Posted: 4/2/2022 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Diphenhydramine (B) has historically been utilized in combination with haloperidol 5mg (5) and lorazepam 2mg (2) in the treatment of acute agitation. The most common rationale for adding diphenhydramine is prevention of EPS, however literature to support this is lacking. A recently published paper examined diphenhydramine/haloperidol/lorazepam combination (B52) vs haloperidol/lorazepam combination therapy (52) to compare the need for additional agitation treatments as a surrogate for clinical efficacy.
This retrospective, multicentered noninferiority study included 400 emergency medicine patients, 200 per treatment arm. On average, the patients were 40 years old, 64% male, and predominantly Caucasian. More patients in the B52 group had psychiatric illness listed as their primary cause for agitation compared to the 52 group. The two most frequently reported substances on urine drug screens, if collected, were amphetamines (35%) and cannabinoid (35.5%).
Results:
-No difference in the use of additional agitation medications within 2 hours
-More patients in the 52 group were noted to receive anticholinergic medications within 2 days, but indications varied and were not associated with EPS treatment
The B52 combination was associated with:
---Increased length of stay 17 h (10-26) vs 13.8 h (9-12), p = 0.03
---Increased use of restraints 43% vs 26.5%, p = 0.001
---Hypotension 16% vs 3.5%, p <0.001
---Use of nasal canula oxygen 3% vs 0%, p < 0.01
The addition of diphenhydramine may not be necessary to prevent EPS in patients receiving haloperidol for agitation and is associated with increased length of stay and adverse events, likely due to its additive sedative properties.
Jeffers T, et al. Efficacy of combination haloperidol, lorazepam, and diphenhydramine vs. combination haloperidol and lorazepam in the treatment of acute agitation: a multicenter retrospective cohort study. J Emerg Med. 2022 Mar 11;S0736-4679(22)00057-9. doi: 10.1016/j.jemergmed.2022.01.009
Category: Pharmacology & Therapeutics
Keywords: Calcium, cardiac arrest (PubMed Search)
Posted: 12/4/2021 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Calcium is commonly administered during cardiac arrest, but there is little data to support or refute its use. The Calcium for Out-of-Hospital Cardiac Arrest trial was a randomized, double-blind, placebo-controlled parallel group study conducted in Denmark. Their EMS system responds to all cardiac arrests with an ambulance and a physician-manned mobile emergency care unit.
Adult patients were included if they had out of-of-hospital (OOH) cardiac arrest and received at least 1 dose of epinephrine. Exclusion criteria were traumatic arrest, known or suspected pregnancy, prior enrollment in the trial, receipt of epinephrine from an EMS unit not in the trial, or a clinical indication for calcium during the arrest (i.e. hyperkalemia or hypocalcemia).
Patients received 735mg calcium chloride dihydrate (5 mmol CaCl –US standard product is 1000mg) or saline control immediately after the first dose of epinephrine. A second dose was administered after the second dose of epinephrine if cardiac arrest ongoing. Teams were blinded to the treatments. The primary outcome was ROSC for at least 20 minutes.
397 patients were randomized (197 calcium, 200 saline). The average age was 68 years old, 70% were male, and over 80% of the cardiac arrests occurred at home, 60% witnessed arrests, and 82% received bystander CPR. Only 25% were in a shockable rhythm. The time to first epinephrine and study drug was approximately 17 minutes and over 70% received two doses.
ROSC rates were low and not statistically different between groups, 19% in the calcium group vs 27% in the saline group. There was no difference in survival to 30d or neurologic function. In the patients who did achieve ROSC in the calcium arm, 74% had hypercalcemia.
Bottom Line: The routine use of calcium in out-of-hospital cardiac arrest is not recommended.
Vallentin MF, et al. Effect of intravenous or intraosseous calcium vs saline on return of spontaneous circulation in adults with out-of-hospital cardiac arrest. JAMA. Published online November 30, 2021. doi:10.1001/jama.2021.20929
Category: Pharmacology & Therapeutics
Keywords: vancomycin, allergies (PubMed Search)
Posted: 6/5/2021 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Vancomycin infusion reactions can manifest as pruritus and an erythematous rash of the neck, face, and torso during or after a vancomycin infusion. This is a histamine reaction caused by degranulation of mast cells and basophils, and can be caused short infusion times <60 min. It is commonly treated with antihistamines and/or a slowing of the infusion rate.
Category: Pharmacology & Therapeutics
Posted: 3/7/2021 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Tranexamic acid (TXA) is an antifibrinolytic medication that has been trialed in previous small studies to treat epistaxis. The data to this point has not reliably shown a reduction in bleeding at 30 minutes, but has demonstrated an increased rate of discharge at 2 hours and a reduction in re-bleeding events.
The NoPAC study was the largest study to date on TXA for epistaxis. It was a double-blind, placebo-controlled, randomized study of TXA in adult patients with persistent atraumatic epistaxis to determine if TXA use decreased the rate of anterior nasal packing. Patients were excluded if they had trauma, out of hospital nasal packing, allergy to TXA, nasopharyngeal malignancy, hemophilia, pregnancy, or if they were referred to ENT.
Eligible patients completed 10 minutes of first aid measures followed by 10 minutes of topical vasoconstrictor application prior to randomization to either placebo of 200mg TXA soaked dental rolls inserted in the nare.
496 patients were enrolled. The average patient was 70 years old with stable vitals 150/80mmHg, HR 80 bpm with >60% on oral anticoagulants.
TXA did not reduce the need for anterior nasal packing: 100 (41.3% placebo) vs 111 (43.7% TXA) OR 1.11 (0.77-1.59). There were no differences in the rates of adverse events.
Bottom Line: TXA does not improve rates of anterior nasal packing for patients with persistent epistaxis.
Reuben A, Appelboam A, Stevens KN, et al. The use of tranexamic acid to reduce the need for nasal packing in epistaxis (NoPAC): Randomized controlled trial. Ann Emerg Med 2021;1-10. https://doi.org/10.1016/j.annemergmed.2020.12.013
Category: Pharmacology & Therapeutics
Posted: 12/5/2020 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Opioid Conversion Updates
Updated in 2018, some clinicians are unaware of the changes to the opioid conversion tables.
|
| 2010 Recommendations |
| 2018 Updates | ||
| Opioid | IV (mg) | PO (mg) |
| IV (mg) | PO (mg) |
| Morphine | 10 | 30 |
| 10 | 25 |
| Fentanyl | 0.1 | NA |
| 0.15 | NA |
| Hydromorphone | 1.5 | 7.5 |
| 2 | 5 |
| Oxycodone | NA | 20 |
| NA | 20 |
When converting between opioids, it is important to remember the following steps:
While online calculators can be helpful, opioid conversions should be done thoughtfully with a full patient assessment to determine the correct conversion for the individual patient.
References:
Category: Pharmacology & Therapeutics
Keywords: nitroglycerin, hypotension, PDE5 inhibitors (PubMed Search)
Posted: 10/3/2020 by Ashley Martinelli
Click here to contact Ashley Martinelli
Nitroglycerin is a potent vasodilator used most commonly for the treatment of angina and ACS. It can also be administered as a continuous infusion for acute management of a hypertensive emergency or sympathetic crashing acute pulmonary edema.
Most are aware of asking men for history of medications for erectile dysfunction (PDE5 inhibitors: sildenafil, tadalafil, vardenafil) but many overlook the fact that men and women may be on these medications chronically for pulmonary hypertension. Men can also be on these medications for the treatment of BPH. Be broad in your history taking and do not limit the discussion to erectile dysfunction or a specific gender.
Drug interaction:
-PDE5 inhibitors prevent the breakdown of cGMP
-Nitrates are nitric oxide donors that increase the production of cGMP
-The combination can lead to excessive vasodilation
If accidentally co-administered:
There is no antidote for this medication error. Support the patient with Trendelenburg positioning, fluid administration, and if needed, vasopressors such as norepinephrine until blood pressure stabilizes.
How long should you wait to administer nitrates after a patient takes a PDE5 Inhibitor?
Sildenafil and vardenafil: 24 h after last dose*
Tadalafil > 48 h after last dose*
*Even if acute ACS event
Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
Category: Pharmacology & Therapeutics
Keywords: esmolol, cardiac arrest, ventricular tachycardia, ventricular fibrillation (PubMed Search)
Posted: 9/5/2020 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
| | Beta-blockade N=22 | Control N= 44 | OR/CI |
| Temporary ROSC, n (%) | 19 (86.4) | 14 (31.8) | OR 14.46, 95% CI 3.63-57.57 |
| Sustained ROSC, n (%) | 13 (59.1) | 10 (22.7) | OR 5.76, 95% CI 1.79-18.52 |
| Survival with neurological function, n (%) | 6 (27.3) | 4 (9.1) | OR 4.42; 95% CI 1.05-18.56 |
Category: Pharmacology & Therapeutics
Keywords: opioid, renal failure, dialysis (PubMed Search)
Posted: 7/6/2020 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Pain management can be challenging in patients with acute or chronic renal failure. Opioid medications should always be used with caution, but some are safer than others. Morphine and codeine specifically should be avoided in these patients due to accumulation of active metabolites that can prolong the duration of effect and adverse events.
| Opioid | Renal Failure Impacts | Renal Failure Recommendation | Dialysis Recommendation |
| Morphine | Active metabolites accumulate | | |
| Codeine | Active metabolites accumulate | | |
| Hydromorphone | Minimal active metabolites | | |
| Oxycodone | Minimal active metabolites | | |
| Fentanyl | No active metabolites | | |
| Methadone | Active metabolites are inactive | | |
Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Mange 2004;28:497-504.
Category: Pharmacology & Therapeutics
Keywords: acute kidney injury (PubMed Search)
Posted: 5/2/2020 by Ashley Martinelli
(Updated: 2/1/2026)
Click here to contact Ashley Martinelli
Short periods of AKI have been linked to prolonged hospitalizations, development of CKD/ESRD and in-hospital mortality. Historically, AKI in the ED has been studied with respect to the receipt of contrast media with little data available on nephrotoxic medications.
A recent 5-year retrospective cohort study sought to determine the impact of prescribing nephrotoxic medications in the ED and the development of AKI within 7 days defined as an increase in SCr of ≥ 0.3 mg/dL or 1.5 x baseline. The categories of potentially nephrotoxic medications included ACE-i/ARB, antibiotics, diuretics, NSAIDs, and other (antifungal, antineoplastic, and antivirals).
Inclusion: Adult patients ≥ 18 years, with an initial and repeat SCr measured 24-168h after the initial test, under admitted or observation status (discharged patients were included if they had a repeat SCr in the time window).
Exclusion: previous hospital or ED visit within 7 days, initial SCr < 0.4 mg/dL, initial SCr > 4.0 mg/dL, missing data, dialysis, or transplant history.
The authors assessed 46,965 hospitalized encounters and found that 13.8% of patients developed AKI. Risk factors included older age, African American patients, history of CHF or CKD, higher initial SCr, and higher complexity and mortality. AKI developed within 48 hours in half of the patients and the reminder did so by 120 hours. Approximately 22% had ≥ 1 potentially nephrotoxic medication administered and 6% had ≥ 2 classes.
Diuretics were associated with the highest risk of AKI (64% increased risk), followed by ACE-i/ARBs (39%), and antibiotics (13%). NSAIDs were not associated with an increased risk. The antibiotics associated with the highest risk of AKI were piperacillin-tazobactam, sulfamethoxazole-trimethoprim, and quinolones.
Bottom Line: Medications prescribed in the ED have an impact on the development of AKI during hospitalization. While these cannot always be avoided, use caution when combining multiple nephrotoxic medications and discontinue therapy early when feasible.
Hinson J, et al. Risk of acute kidney injury associated with medication administration in the emergency department. J Emerg Med. 2020;58(3): 487-496.
