Category: Critical Care
Keywords: Shock, hemodynamics, RIAD, Renal interlobar artery doppler, Resistive Index (PubMed Search)
Posted: 6/16/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
Renal Resuscitation using Renal Interlobar Artery Doppler (RIAD)
Shocked patient…. check! Adequate volume resuscitation…. check! Vasopressors.… check! Mean arterial pressure (MAP) > 65 mmHg….. check! Adequate urine output…. Wait, why isn’t my patient making urine?
As we begin to understand more about shock, hemodynamics, and the importance of perfusion over the usual macrocirculatory goals (MAP > 65), finding ways to assess regional blood flow is critical. A recent study examined the effect of fluid administration on renal perfusion using renal interlobar artery Doppler (RIAD) to assess the interlobar resistive index (RI). See how to perform a RIAD here.
They also recorded the fluid challenge’s effect on the traditional hemodynamic measurements of MAP and pulse pressure (PP) then observed the patient’s urine output (as a clinical marker of perfusion). The authors reported 3 key findings:
Bottom Line: The use of ultrasound to determine intrarenal hemodynamics is an interesting strategy to guide renal resuscitation in the shocked patient. There is mixed data on the use of RIAD, however this study could explain the findings of SEPSISPAM and also addresses the growing concern that traditional hemodynamic goals may be inadequate resuscitation targets.
References
For more critical care & resuscitation pearls, follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: Pulmonary Embolism (PubMed Search)
Posted: 5/19/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
Advances in Catheter-Directed Therapy for Acute PE - The PERFECT Registry
Earlier this month, initial results from the multicenter PERFECT registry (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) were released. In this study, 101 consecutive patients with massive or submassive PE were prospectively enrolled to receive early catheter-directed therapy.
Inclusion criteria:
Therapy provided:
Outcomes: Clinical success (stabilization of hemodynamics, improvement in pulmonary hypertension and/or right heart strain, and survival to discharge) was achieved in 86% of patients with massive PE and 97% of patients with submassive PE. There were no major procedure-related complications or major bleeding events.
Bottom Line: In patients with massive or submassive pulmonary embolism, there is growing evidence that early catheter-directed therapy may become first-line therapy for selected patients.
1. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results from a Prospective Multicenter Registry. Chest. 2015 (ePub April, 2015)
For more Critical Care pearls, follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: large hemispheric infarct, acute ischemic infarct, stroke (PubMed Search)
Posted: 4/20/2015 by John Greenwood, MD
(Updated: 4/21/2015)
Click here to contact John Greenwood, MD
Updates in the Management of Large Hemispheric Infarction
Large hemispheric infarctions (LHI) are estimated to occur in 2-8% of all hospitalized ischemic strokes and 10 15% of all MCA territory infarcts. LHI carry high rates of morbidity and mortality, in fact, if left untreated associated cerebral edema can rapidly progress to transtentorial herniation and death in 40 80% of patients.
Recognized risk factors for progressive cerebral edema include:
Evidence based medical strategies for LHI include:
Prophylactic hemicraniectomy
Bottom Line: Early recognition of large hemispheric stroke is critical as it is associated with a high rate of morbidity and mortality. Aggressive medical management and early neurosurgical involvement may improve outcomes.
References
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: mechanical ventilation, ARDS, PEEP (PubMed Search)
Posted: 3/24/2015 by John Greenwood, MD
(Updated: 2/1/2026)
Click here to contact John Greenwood, MD
Stop looking for the “Best PEEP”, aim for a “Better PEEP”
Mechanical ventilation settings in the patient with acute respiratory distress syndrome (ARDS) need to provide adequate gas exchange and prevent ventilator induced lung injury (VILI). Positive end-expiratory pressure (PEEP) is often prescribed with consideration of the patient’s FiO2 requirement, estimated chest wall compliance, and hemodynamic tolerance.
So what is the best strategy for PEEP prescription?
In a recent review, Gattinoni & colleagues analyzed a number of the recent studies examining PEEP optimization. In this paper, the authors conclude that there is no “Best PEEP,” and regardless of the level chosen there will be some degree of intratidal recruitment-derecruitment and VILI. They go on to recommend a PEEP prescription strategy that reflects the severity of ARDS using the patient’s PaO2/FiO2 or P/F ratio.
Bottom line: There is no “Best PEEP” however, a “Better PEEP” is one that is primarily tailored to the severity of the patient’s ARDS, but also compensates for chest wall resistance and minimizes hemodynamic compromise.
References
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: CVP (PubMed Search)
Posted: 2/24/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
The Role of the CVP in a Post- “7 Mares” Era
The role for using central venous pressure (CVP) as a measure of volume responsiveness has largely fallen out of favor over the years.1 There are certainly better indices for fluid responsiveness, but don’t be fooled – the CVP isn’t a one trick pony. In fact, a high or rapidly rising CVP should raise a significant concern for impending cardiovascular collapse.
Consider the following differential diagnosis in the patient with an abnormally high or rising CVP ( >10 cm H2O).
Bottom Line: In a time where the utility of the CVP has been largely dismissed, remember that an abnormal CVP offers great deal of information beyond a simple measure of volume status.
References
Follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Keywords: Methanol, toxicology, methanol toxicity, critical care (PubMed Search)
Posted: 1/20/2015 by John Greenwood, MD
(Updated: 1/30/2015)
Click here to contact John Greenwood, MD
Extracorporeal Treatment Strategies for Acute Methanol Poisoning (When to Dialyze)
Methanol toxicity is classically included in the differential for the intoxicated patient presenting to the ED. Add a negative EtOH level, anion/osmolar gap, blindness and you have yourself a slam dunk diagnosis. The goal is to stop the liver from metabolizing methanol to formic acid. Outside of fomepizole (or old school ethanol therapy), dialysis is often discussed, but when should you actually get the nephrologist on the phone?
This month the Extracorporeal Treatments in Poisoning Workgroup released a systematic review and consensus statement to help clinicians decide when to pull the HD trigger. Their suggestions are below.
When to start HD:
Which Modality: Intermittent HD (IHD) should be used over continuous renal replacement therapies (CRRT), as you can clear the toxin faster with higher HD flows.
When to stop HD: Extracorporeal treatment can be terminated when the methanol concentration is less than 200 mg/L or 6.2 mmol/L and a clinical improvement is observed.
Bottom Line: Consider early hemodialysis in most patients presenting with methanol toxicity. Clinical exam and routine lab testing will likely provide enough information to determine the need for IHD, but specific methanol levels can be helpful to guide adjunctive treatment options.
Reference
Roberts DM, Yates C, Megarbane B, et al. Recommendations for the Role of Extracorporeal Treatments in the Management of Acute Methanol Poisoning: A Systematic Review and Consensus Statement. Crit Care Med. 2015;43(2):461-472.
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 12/30/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Cartoons Kill: A new high-risk patient for critical illness & death
This past month, the BMJ published an impressive retrospective review that analyzed nearly 80 years of data to find that animated characters in children’s films are in fact at a very high-risk for death when compared to characters in adult dramas.
Films ranged from 1937 (Snow White) to 2013 (Frozen) and were compared against the two highest gossing dramatic films in that same year. The authors found that nearly two thirds of the children’s animated films contained an on-screen death of an important character compared to only half in adult dramas.
Fatalities were most commonly the result of:
Other high-risk animated characters include the parents of the protagonist (17.8% mortality) and nemeses (28.9% mortality). Median survival time was approximately 90 minutes (much less than the usual ED LOS!)
Notable early on-screen deaths included Nemo’s mother being eaten by a barracuda 4 minutes into Finding Nemo, Tarzan’s parents being killed by a leopard 4 minutes into Tarzan, and Cecil Gaines’ father being shot in front of him 6 minutes into The Butler.
The author’s intention was to point out the psychological impact of death on young children, but I think the authors also highlight an important, high-risk patient population that could present to your ED.
Bottom Line: Animated characters should be aggressively resuscitated and strongly considered for admission to a higher level of care should they present to your ED, as they appear to be at high-risk for death and rapid decompensation.
May all of you have a happy and safe 2015!
Reference
1. Colman I, Kingsbury M, Weeks M, et al. CARTOONS KILL: casualties in animated recreational theater in an objective observational new study of kids' introduction to loss of life. BMJ. 2014;349:g7184.
Follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 12/2/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Dynamic Measures of Intravascular Volume Assessment
The resuscitation of a patient in shock often requires the administration of intravenous fluid. Excessive fluid resuscitation can lead to worsening pulmonary edema, systemic edema, acid-base disturbances, as well as many other complications. There are a myriad of techniques to try and figure out if the patient needs more intravascular volume, but each has it’s pitfalls.
Recently, experts have recommend that we move away from using static measures of preload assessment such as central venous pressure (CVP) and instead focus on using dynamic measures for volume responsiveness.
Volume Responsiveness Defined: An increase of stroke volume of 10-15% after a 500 mL IV crystalloid bolus over 10-15 minutes.
Below is a chart describing key values, requirements, and contraindications for each of these dynamic measures of non-invasive intravascular volume assessment.
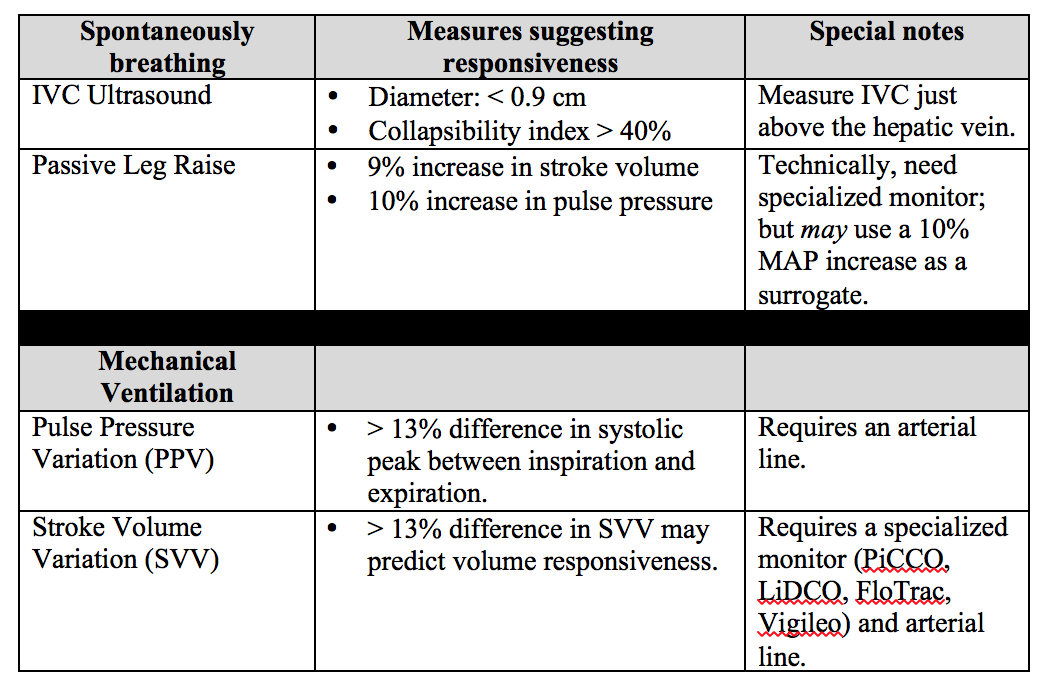
Important notes: PPV and SVV require the patient to be intubated with controlled tidal volumes. Arrhythmias and right heart failure make many of these measures invalid (except for PLR). Other methods of assessment not discussed include systolic pressure variation, left ventricular outflow track velocity time integral (LVOT VTI), and end-expiratory occlusion pressure (EEO).
Bottom Line: None of these measures are perfect and shouldn't be used in isolation to determine if the patient’s “tank is full”. Combine clinical judgment with these measures to get a best estimate of whether or not to give that next fluid bolus.
Reference
1. Enomoto TM, Harder L. Dynamic indices of preload. Crit Care Clin. 2010;26(2):307-21,
Follow Me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: Airway, critical care, RSI, rapid sequence intubation (PubMed Search)
Posted: 11/3/2014 by John Greenwood, MD
(Updated: 11/4/2014)
Click here to contact John Greenwood, MD
Back 2 Basics Series: Your Simple RSI Checklist - SOAP ME
The use of a checklist during high stress medical procedures is often recommended. Rapid sequence intubation (RSI) is a classic situation where having a checklist can ensure adequate preparation however, if you don’t have a checklist – this simple mnemonic will make sure you are well prepared for a successful intubation.
| Mnemonic – “SOAP ME” | |
|---|---|
| Suction |
|
| Oxygen |
|
| Airways |
|
| Positioning |
|
| Monitors & Meds |
|
| EtCO2 & other Equipment |
|
The SOAP ME mnemonic is a quick and useful technique to remember only the basics of airway management and preparation. Always remember to also assign roles to team members and communicate clearly to maximize your chances of success.
Category: Critical Care
Posted: 10/6/2014 by John Greenwood, MD
(Updated: 10/7/2014)
Click here to contact John Greenwood, MD
The ARISE Trial
Early, aggressive resuscitation and attention to detail are essential element of managing critically ill patients. This past week the ARISE trial was published - a 2nd large, randomized control study to examine the benefit of protocolized vs. usual care in patients with severe sepsis and septic shock.
What were the main findings? After enrolling 1,600 patients who presented to the ED in severe sepsis or septic shock:

Bottom Line: Resuscitation goals for the patient with septic shock should include:
Additional therapeutic goals should be made on a patient by patient basis. Reassess your patient frequently, pay attention to the details, and you will improve your patient’s mortality.
Suggested Reading
Follow Me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 9/8/2014 by John Greenwood, MD
(Updated: 9/9/2014)
Click here to contact John Greenwood, MD
Goal-Directed Resuscitation During Cardiac Arrest
Focusing on high-quality CPR is by far one of the most effective methods to ensure your arrested patient has the best chance to survive. However, emerging evidence suggests that there are additional goals that we should try and accomplish during our resuscitation.
As we continue to move toward goal-directed resuscitation strategies, optimizing coronary perfusion pressure (CPP) may be our next target in “personalizing” the care we provide to those in cardiac arrest.
A recent AHA consensus statement recommended the following physiologic goals during cardiac arrest care:
Each of these variables can give the provider valuable feedback about how their patient is responding to their resuscitation. Some argue that the DBP target should be much higher (>35 mmHg), with the caveat that pharmacologic optimization can only occur once high quality CPR is confirmed. The goal should always be to minimize the use of epinephrine whenever possible!
Bottom Line: During your next cardiac arrest resus, consider using a goal-directed strategy by monitoring the patient’s CPP, DBP, & EtCO2 to determine the effectiveness of your resuscitation.
Suggested Reading
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Keywords: cardiomyopathy, sepsis, septic shock, pressors, inotropes, epinephrine, norepinephrine, dobutamine (PubMed Search)
Posted: 8/12/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Should I Give My Patient with Septic Cardiomyopathy Fluids?
The incidence of acute LV dysfunction in septic shock is estimated to occur in 18 - 46% of patients within the first 24 hours of shock. Unlike the "classic" pattern of cardiogenic shock where LV filling pressure is high, in septic shock there are normal or low LV filling pressures.
Three therapeutic options should be strongly considered in the patient with a septic cardiomyopathy [CM]:
Recommended Reading
Vieillard-Baron, A. Septic cardiomyopathy. Ann Intensive Care. 2011; 1:6.
Follow me on Twitter @JohnGreenwoodMD
For more critical care pearls & education check out http://www.marylandccproject.org
Category: Critical Care
Posted: 7/14/2014 by John Greenwood, MD
(Updated: 7/15/2014)
Click here to contact John Greenwood, MD
Patient Positioning During Mechanical Ventilation
In any patient with acute respiratory failure, it is extremely important to consider patient positioning after initiating mechanical ventilation. Both ventilation (V) and perfusion (Q) of the lungs can be significantly altered by manipulating the way you position your patient.
Follow me on Twitter @JohnGreenwoodMD
For more critical care pearls & education check out http://www.marylandccproject.org
Category: Critical Care
Keywords: Thrombelastography, TEG, ROTEM, Hemorrhagic Shock (PubMed Search)
Posted: 6/13/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Thrombelastography for Management of Non-Traumatic Hemorrhagic Shock
The use of thrombelastography (TEG, ROTEM) has traditionally been utilized and studied in the management of acute coagulopathy of trauma (ACoT) developed by patients in hemorrhagic shock secondary to trauma.
Functional coagulation tests such as the TEG may provide valuable information when resuscitating the hemorrhaging patient, especially if there is any concern for an underlying coagulopathy.
The following is a TEG recently returned during the resuscitation of a 60 y/o male with a history of HCV cirrhosis presenting with hemorrhagic shock secondary to a massive upper GIB. The University's Massive Transfusion Protocol was promptly activated and at this point, the patient had received approximately 4 units of PRBCs & FFP along with 1 liter of crystalloid. His Hgb was 5, PT/PTT/INR were undetectable, and his fibrinogen was 80.
Below is a table that simplifies the treatment, based on the test's abnormalities:
After reviewing the initial TEG, all perameters were abnormal in addition to the presence of significant fibrinolysis. The patient was given an additional 4 units of FFP, DDAVP, cryoprecipitate, a unit of platelets, and aminocaproic acid. The patient still required significant resuscitation, however bleeding had significantly decreased as well has his pressor requirement. Below is the patient's follow-up TEG 2 hours later.
There is growing enthusiasm for the use of functional coagulopathy testing in the patient with hemorrhagic shock. Early resuscitation with blood products as your fluid of choice with limited fluid administration while arranging for definitive source control are critical, but also consider early thrombelastography to detect additional causes for uncontrolled hemorrhage.
References
Follow Me On Twitter: @JohnGreenwoodMD
email: johncgreenwood@gmail.com
Category: Critical Care
Keywords: Carbapenem Resistant Organisms, CRE, Pseudomonas, Infectious Diseases, Antimicrobial Stewardship (PubMed Search)
Posted: 5/15/2014 by John Greenwood, MD
(Updated: 5/20/2014)
Click here to contact John Greenwood, MD
We've all heard Dr. Bryan Hayes warn us that, "Vanc & Zosyn is NOT the Answer for Everything" but things just got a little more serious, on a whole 'nother level...
Within the past few months, 2 cases of NDM-producing carbapenem-resistant pseudomonas have been reported in the area - one in Delaware and one in Pennsylvania. Previously, the only reported cases were found in Europe.
Few treatment options are currently available for carbapenem resistant organisms.
Appear to have retained some in vitro activity against these organisms, but are generally used as, "drugs of last resort".
Know it exists, take a good history, & know your local antibiogram. Prior to selecting a broad spectrum antimicrobial regimen, try to obtain previous antimicrobial culture data for patients with resistant organism infectious risk factors.
For more critical care pearls, follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Keywords: intubation, neurocritical care, mechanical ventilation, direct laryngoscopy, video laryngoscopy (PubMed Search)
Posted: 4/20/2014 by John Greenwood, MD
(Updated: 4/22/2014)
Click here to contact John Greenwood, MD
Direct vs. video laryngoscopy in the patient with an acute TBI
Hypoxia and hypotension are considered the "lethal duo" in patients with traumatic brain injury. In a recent randomized control trial (by our own Dr. Dale Yeatts at the Shock Trauma Center) mortality outcomes were compared between 623 consecutive patients who were intubated with either direct laryngoscopy (DL) or video laryngoscopy (VL). Here is what they found:
1. No significant difference in mortality for all comers (Primary Outcome)
2. In the subset of patients with severe head injuries, there was:
There is a reasonable amount of literature that shows hypoxia and hypotension significantly contribute to morbidity & mortality in the TBI patient, and a growing body of literature that suggests intubation with VL takes longer than DL.
Bottom Line: When choosing a method of intubation for the TBI patient, remember the "Lethal Duo" and consider direct laryngoscopy with manual inline stabilization first.
Reference
Yeatts DJ, Dutton RP, Hu PF, et al. Effect of video laryngoscopy on trauma patient survival: a randomized controlled trial. J Trauma Acute Care Surg. 2013;75(2):212-9.
Follow me on Twitter @JohnGreenwoodMD
Email: johncgreenwood@gmail.com
Category: Critical Care
Keywords: ARDS, Nitric Oxide, acute respiratory failure, mechanical ventilation (PubMed Search)
Posted: 3/23/2014 by John Greenwood, MD
(Updated: 3/26/2014)
Click here to contact John Greenwood, MD
Nitric Oxide appears to have NO role in ARDS
Background: The use of inhaled nitric oxide (iNO) in acute respiratory distress syndrome (ARDS) & severe hypoxemic respiratory failure has been thought to potentially improve oxygenation and clinical outcomes. It is estimated that iNO is used in up to 14% of patients, despite a lack of evidence to show improved outcomes.
Mechanism: Inhaled NO works as a selective pulmonary vasodilator which has been found to improve PaO2/FiO2 by 5-13%, but is costly ($1,500 - $3,000 per day) and increases risk of renal failure in the critically ill.
Study: A recent systematic review analyzed 9 different RCTs (N=1142) and compared mortality between those with severe (PaO2/FiO2 < 100) and less severe (PaO2/FiO2 > 100) ARDS and found that iNO does not reduce mortality in patients with ARDS, regardless of the severity of hypoxemia.
Bottom Line: Inhaled NO is an intriguing option for the treatment of refractory hypoxemic respiratory failure, however there does not appear to be a mortality benefit to justify it's high cost and potentially negative side effects. In the ED, it is important to focus on appropriate lung protective ventilation strategies (TV: 6-8 cc/kg IBW) and maintaining plateau pressures < 30 cm H2O in the initial stages of ARDS to prevent ventilator induced lung injury while awaiting ICU admission.
Reference
Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014;42(2):404-12. [PMID: 24132038]
Follow me on Twitter (@JohnGreenwoodMD)
Category: Critical Care
Keywords: INTERACT 2, ATACH II, Intracranial Hemorrhage, Hypertensive Emergency, Hemodynamics (PubMed Search)
Posted: 2/24/2014 by John Greenwood, MD
(Updated: 2/25/2014)
Click here to contact John Greenwood, MD
Intensive BP Control in Spontaneous Intracranial Hemorrhage
Managing the patient with hypertensive emergency in the setting of spontaneous intracerebral hemorrhage (ICH) is often a challenge. Current guidelines from the American Stroke Association are to target an SBP of between 160 - 180 mm Hg with continuous or intermittent IV antihypertensives. Continuous infusions are recommended for patients with an initial SBP > 200 mm Hg.
An emerging concept is that rapid and aggressive BP control (target SBP of 140) may reduce hematoma formation, secondary edema, & improve outcomes.
Recently published, the INTERACT 2 trial (n=2,829) compared intensive BP control (target SBP < 140 within 1 hour) to standard therapy (target SBP < 180) found:
Study flaws: Patients treated with multiple drugs - combinations of urapadil, labetalol, nicardipine, nitrates, hydralazine, and diuretics. Management variability away from protocol seemed high. (Interesting editorial)
A Post-hoc analysis of the INTERACT 2 published just this month suggests that large fluctuations in SBP (>14 mmHg) during the first 24 hours may increase risk of death & major disability at 90 days.
Bottom Line: INTERACT 2 was a large RCT but not a great study (keep on the look out for ATACH II). However, in patients with spontaneous ICH, consider early initiation of an antihypertensive drip (preferably nicardipine) in the ED to reduce blood pressure fluctuations early with a target SBP of 140 mmHg.
Follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Keywords: arterial line, catheter related blood stream infections (PubMed Search)
Posted: 1/20/2014 by John Greenwood, MD
(Updated: 1/21/2014)
Click here to contact John Greenwood, MD
Arterial Catheter-Related Blood Stream Infections
Whether arterial lines are a potential source of catheter-related blood stream infections (CRBSIs) is highly-debated; however, based on a recent systematic review they are an under recognized and significant source of CRBSIs.
Bottom Line(s)
Follow me on twitter @medicalgraffiti
Category: Critical Care
Keywords: Left Ventricular Assist Device, LVAD, Critical Care, Cardiology, Heart Failure, Thrombosis, LVAD Complications (PubMed Search)
Posted: 12/31/2013 by John Greenwood, MD
Click here to contact John Greenwood, MD
VAD thrombosis: A Must Know VAD Complication
The HeartMate left ventricular assist device (LVAD) is one of the most frequently placed LVADs today. Originally, it was thought to have a lower incidence of thrombosis due to its mechanical design. However, a recent multi-center study published in the NEJM reported a dramatic increase in the rate of thrombosis since 2011 in the HeartMate II device. The report found:
An increase in pump thrombosis at 3 months after implantation from 2.2% to 8.4%
The median time from implantation to thrombosis was 18.6 months prior to March 2011, to 2.7 months after.
Pump thrombosis is a major cause of morbidity and mortality (up to almost 50%!!) and is a can't miss diagnosis. It's important to keep thrombosis on the differential for any VAD patient presenting with:
Power spikes or low pump flow alarms on the patient's control box
Pump (VAD) failure
Recurrent/new heart failure
Altered mental status
Hypotension (MAP < 65)
Signs of peripheral emboli (including acute CVA)
Useful lab findings suggestive of thrombosis include:
Evidence of hemolysis
LDH > 1,500 mg/dL or 2.5-3 times the upper limit of normal
Hemoglobinuria
Elevated plasma free hemoglobin
Bottom Line: In the patient with suspected VAD thrombosis, it is important to contact the patient's VAD team immediately (CT surgeon, VAD coordinator/nurse, VAD engineer). Treatment should begin with a continuous infusion of unfractionated heparin, while other treatment options can be discussed with the VAD team.
Starling RC, Moazami N, Silvestry SC, et al. Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis. N Engl J Med. 2013.
Follow Me on Twitter: @medicalgraffiti
Email: johncgreenwood@gmail.com
