Category: Critical Care
Posted: 12/29/2015 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Acute Chest Syndrome
Cecchini J, Fartoukh M. Sickle cell disease in the ICU. Curr Opin Crit Care 2015; 21:569-75.
Category: Critical Care
Keywords: Critical care, Trauma, TBI, ICP, hypothermia (PubMed Search)
Posted: 12/22/2015 by Daniel Haase, MD
Click here to contact Daniel Haase, MD
The EuroTherm3235 Trial was a randomized, multi-center trial to study hypothermia (32-35oC) in severe, traumatic brain injury1:
1. Andrews PJ, Sinclair HL, et al; Eurotherm3235 Trial Collaborators. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N Engl J Med. 2015 Dec 17;373(25):2403-12. doi: 10.1056/NEJMoa1507581. Epub 2015 Oct 7. PubMed PMID: 26444221.
2. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 Suppl 1:S1-106. PubMed PMID: 17511534.
Category: Critical Care
Keywords: plasmalyte, normal saline, fluid, critical care, fluid resuscitation (PubMed Search)
Posted: 12/8/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
The Bottom Line: This was a nicely designed study to evaluate the safety of both fluids. It does suggest that either fluid type is for the most part OK. But in patients requiring hefty fluid boluses, we should be cautious in what type of fluid we choose.
Category: Critical Care
Posted: 12/1/2015 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Mechanical Ventilation for Septic Patients in Resource-Limited Settings
Neto AS, Schultz MJ, Festic E. Ventilatory support of patients with sepsis or septic shock in resource-limited settings. Intensive Care Med 2016:42:100-3.
Category: Critical Care
Keywords: COPD, respiratory failure, antibiotics, ICU (PubMed Search)
Posted: 11/24/2015 by Daniel Haase, MD
Click here to contact Daniel Haase, MD
--The role of antibiotics in acute exacerbations of COPD remains controversial in many settings. However, a recent Cochrane review concludes that antibiotics have "large and consistent" benefit in ICU admissions [1]:
--However, patients on antibiotics had increased side effects, are at risk for increased drug-drug interaction (think azithromycin/levofloxacin), and the effect on multi-drug resistance is unclear.
--GOLD Guidelines are a bit more liberal with their recommendations for antibiotics [2], recommending antibiotics based on symptoms or in patients needing mechanical support.
--TAKEAWAY -- if your patient needs BiPAP or ICU, they should also get antibiotics!
1. Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 Dec 12;12:CD010257. doi: 10.1002/14651858.CD010257. Review. PubMed PMID: 23235687
2. http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2015_Feb18.pdf
Category: Critical Care
Keywords: fungal infections, candida, candidiasis (PubMed Search)
Posted: 11/10/2015 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
Risk factors for invasive candidal infections
Bart Jan Kullberg, M.D., Ph.D., and Maiken C. Arendrup, M.D., Ph.D.
N Engl J Med 2015; 373:1445-1456October 8, 2015DOI: 10.1056/NEJMra1315399
Category: Critical Care
Posted: 11/3/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Pain Management in the Critically Ill Patient
Sigakis MJG, Bittner EA. Ten myths and misconceptions regarding pain management in the ICU. Crit Care Med 2015; 43:2468-2478.
Category: Critical Care
Posted: 10/20/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
There is more than the standard preparations of plasma, platelets, and PRBCs in the blood bank. Certain patients will require these specialized preparations when a transfusion is required. Here are three to know:
Follow me on Twitter (@criticalcarenow)
Category: Critical Care
Keywords: central line, cvc (PubMed Search)
Posted: 10/13/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Parienti et al. INtravascular complications of central venous catherization by insertion site. N ENGL J MED 373;13. Sept 24, 2015
Category: Critical Care
Keywords: Aortic dissection, STEMI, cardiac tamponade, aortic insufficiency, echocardiography (PubMed Search)
Posted: 9/30/2015 by Daniel Haase, MD
Click here to contact Daniel Haase, MD
Classically, aortic dissection presents as tearing or ripping chest pain that radiates to the back in a HYPERtensive patient.
However, type A aortic dissections can quickly become HYPOtensive due to any the primary cardiac complications from retrograde dissection into:
Bedside echo can't rule out aortic dissection, but it can help rule in the diagnosis (figure 1) or complications (figure 2) at times.
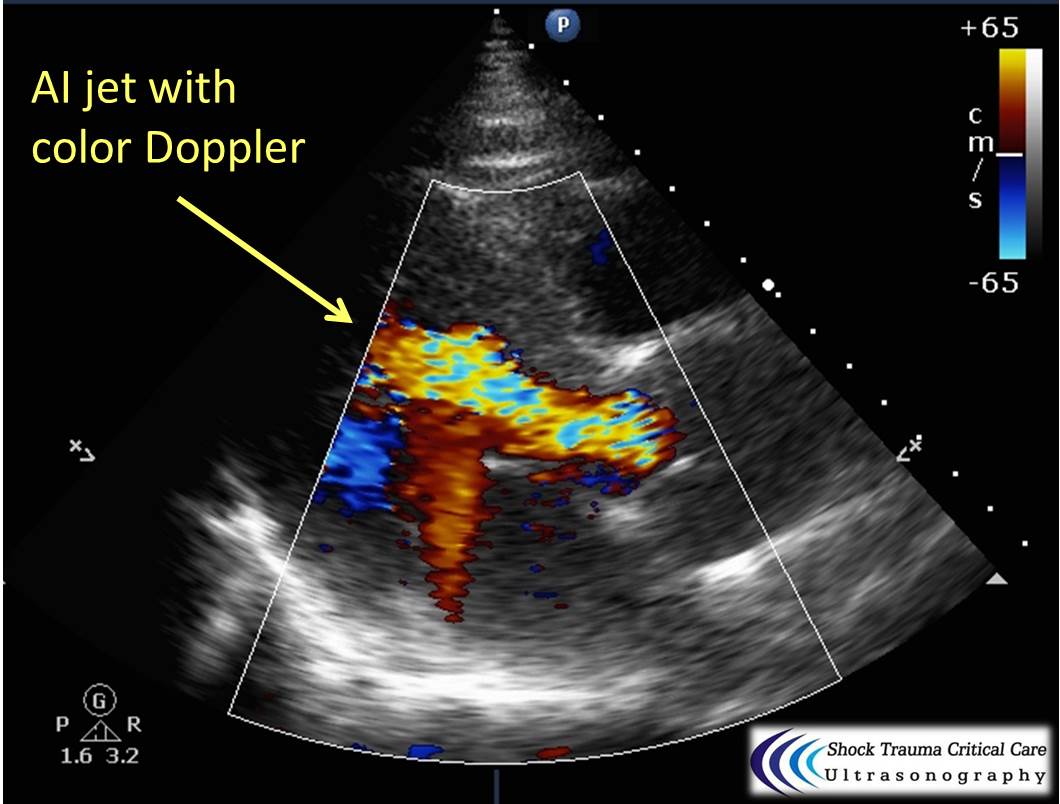
Category: Critical Care
Posted: 9/22/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
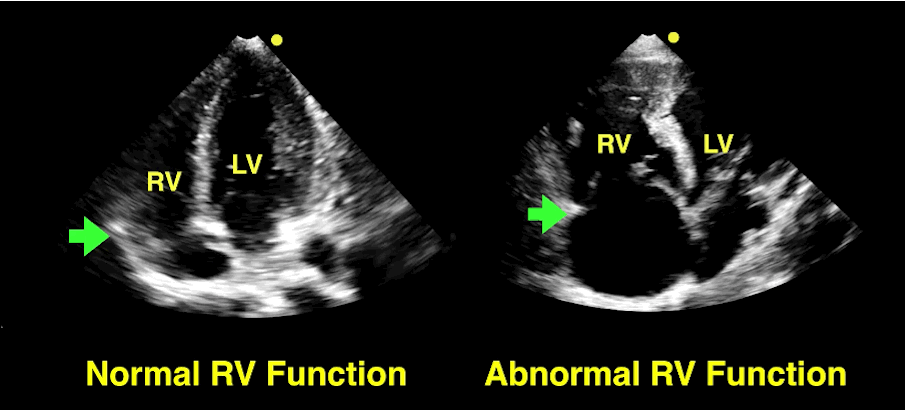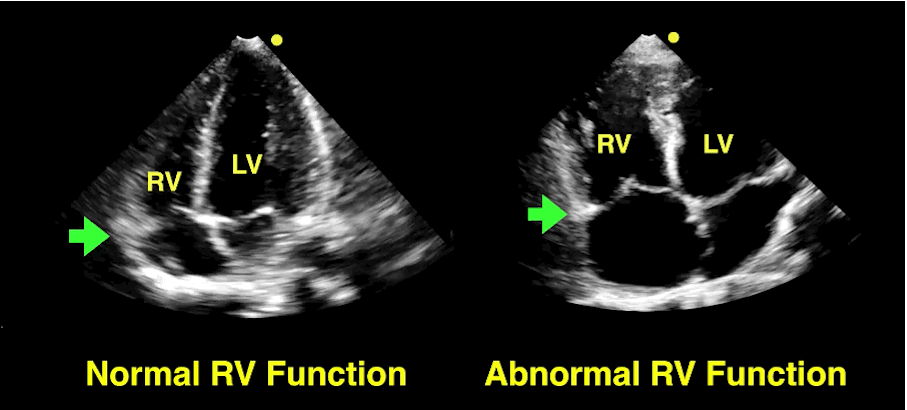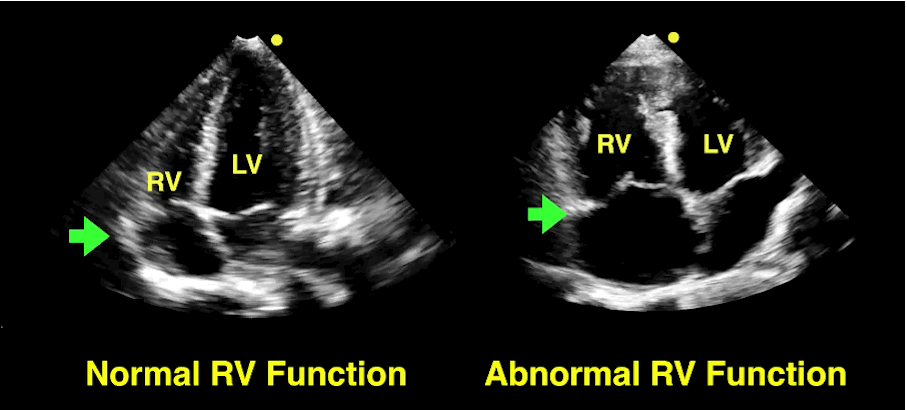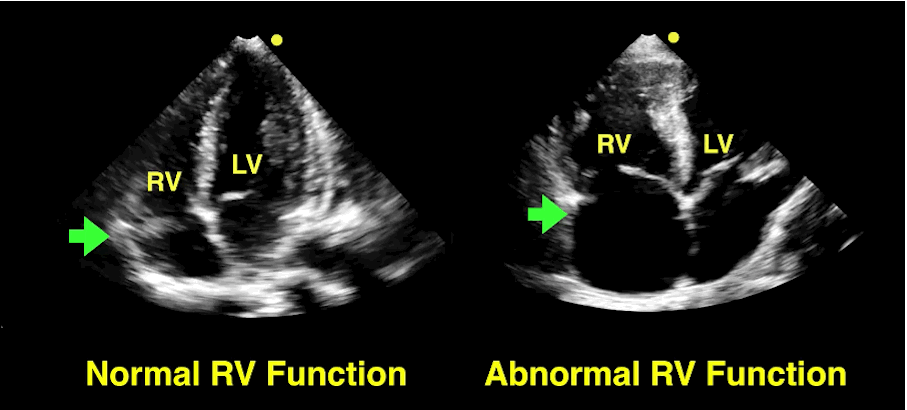
Follow me on Twitter (@criticalcarenow)
Category: Critical Care
Keywords: Simv, critical care, ventilator (PubMed Search)
Posted: 9/15/2015 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
SIMV (Synchronized intermittent mandatory ventilation)
Category: Critical Care
Posted: 9/8/2015 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Hyperoxia in the Critically Ill
Helmerhorst HJF, et al. Association between arterial hyperoxia and outcomes in subsets of critical illness: A systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med 2015; 43:1508-19.
Category: Critical Care
Keywords: Paracentesis, cirrhosis, ascites, critical care (PubMed Search)
Posted: 9/1/2015 by Daniel Haase, MD
Click here to contact Daniel Haase, MD
Your ESLD patient is hypotensive with a tense abdomen, and he needs a paracentesis!
--ALWAYS use ultrasound to localize a fluid pocket [Fig 1]! Take the time to use color Doppler to look for underlying abdominal wall varices [Fig 2]. Cirrhotic patients frequently have abnormal abdominal wall vasculature [1-2].
--Hemorrhage from paracentesis is exceedingly rare, and reversal of mild coagulopathy probably isn't that important [3-4].
--In hypotensive patients, consider placement of a small pigtail catheter for slow, continuous drainage (e.g. 8.3F pericardiocentesis catheter) instead of large-volume paracentesis. Non-tunneled catheter infection risk goes up after 72h [5].
--Albumin replacement improves mortality and incidence of renal failure in patients with SBP or other infection [6-7].
1. Hatch N, Wu TS, Barr L, Roque PJ. Advanced ultrasound procedures. Crit Care Clin. 2014 Apr;30(2):305-29, vi. doi: 10.1016/j.ccc.2013.10.005. Epub 2013 Dec 4. Review. PubMed PMID: 24606778.
2. Thomsen TW, Shaffer RW, White B, Setnik GS. Videos in clinical medicine. Paracentesis. N Engl J Med. 2006 Nov 9;355(19):e21. Erratum in: N Engl J Med. 2007 Feb 15;356(7):760. PubMed PMID: 17093242.
3. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005 Mar 1;21(5):525-9. PubMed PMID: 15740535.
4. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991 Feb;31(2):164-71. PubMed PMID: 1996485.
5. Nadir A, Van Thiel DH. Frequency of peritoneal infections among patients undergoing continuous paracentesis with an indwelling catheter. J Ayub Med Coll Abbottabad. 2010 Jan-Mar;22(1):37-41.
6. Kwok CS, Krupa L, Mahtani A, Kaye D, Rushbrook SM, Phillips MG, Gelson W. Albumin reduces paracentesis-induced circulatory dysfunction and reduces death and renal impairment among patients with cirrhosis and infection: a systematic review and meta-analysis. Biomed Res Int. 2013;2013:295153. doi: 10.1155/2013/295153. Epub 2013 Oct 8. Review. PubMed PMID: 24222902; PubMed Central PMCID: PMC3816020.
7. Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Gin s P, Rod s J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999 Aug 5;341(6):403-9. PubMed PMID: 10432325.
Category: Critical Care
Posted: 8/25/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
The RV is a low-pressure chamber that doesn’t tolerate acute increases in pulmonary pressures (e.g., ARDS, pulmonary embolism, etc.); acute increases can lead to RV dysfunction / failure
Managing RV dysfunction requires a three-pronged approach:
Follow me on Twitter (@criticalcarenow)
Category: Critical Care
Keywords: ventilation, prvc (PubMed Search)
Posted: 8/18/2015 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
Pressure Regulated Volume Control (PRVC)
Here are some basic pearls about PRVC Ventilation
Benefits: minimum PIP, guaranteed tidal volume, patient can trigger more breaths, improved oxygenation, breath by breath changes
Category: Critical Care
Posted: 8/11/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Is It Really ARDS?
Guerin C, et al. The ten diseases that look like ARDS. Intensive Care Med 2015; 41:1099-1102.
Category: Critical Care
Keywords: Anion gap, acidosis, metabolic acidosis, ingestion, critical care (PubMed Search)
Posted: 8/4/2015 by Daniel Haase, MD
Click here to contact Daniel Haase, MD
Ever forget all the things that make up MUDPILES in your AG acidosis differential?
Instead, consider the less-complicated mnemonic "KILR"!
K Ketoacidosis (diabetic, alcoholic, starvation)
I Ingestion (salicylate, acetaminophen, methanol, ethylene glycol, CO, CN, iron, INH)
L Lactic acidosis (infection, hemorrhage, hypoperfusion, alcohol, metformin)
R Renal (uremia)
Once you rule out the KLR causes, begin to consider ingestion or a tox source as your source. Remember that many of the listed ingestions can also cause a lactic acidosis.
For more acid/base pearls in greater detail:
http://lifeinthefastlane.com/ccc/anion-gap/
http://emcrit.org/wp-content/uploads/acid_base_sheet_2-2011.pdf (from emcrit.org)
Category: Critical Care
Posted: 7/28/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
It's July, that means new doctors are learning to do central-lines...here's a quick video with some quick pearls on how to do that. Enjoy!
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: drowning, critical care, swimming, swim, water (PubMed Search)
Posted: 7/21/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Care of Drowning Patients in the ED
Szpillman D et al. Current Concepts: Drowning. NEJM 2012;366:2102-2110.
