Category: Critical Care
Posted: 7/14/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Blood Pressure Management in Severe Preeclampsia
Leone M, Einav S. Severe preeclampsia: what's new in intensive care? Intensive Care Med 2015; 41:1343-6.
Category: Critical Care
Keywords: tlc, triple lumen, cordis, catheter, central line, icu, critical care (PubMed Search)
Posted: 6/30/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
With a new academic year starting, it is important to review some details on central lines
Complications of central lines (TLC-Triple lumen catheter)
Avoiding infections: hand hygiene, chlorhexidine skin antisepsis, maximal barrier precautions, remove unnecessary lines, full gown and glove w/ mask and sterile technique.
Catheter position: 16-18cm for Right sided and 18-20 cm for Left sided. But can vary based on height, neck length, and catheter insertion site. Approximate length based on these factors.
Flow rates: Remember that putting in a central line does not necessarily improve your flow rates in resuscitation
16 G IV: 220 ml/min
Cordis/introducer sheath: 126 ml/min
18 G IV: 105 ml/min
16G distal port TLC: 69 ml/min
Ports (Can vary with type of catheter)
1. Distal exit port (16G)
2. Middle port (18G)
3. Proximal port (18G)
Arterial puncture: hold pressure for 5 mins and evaluate for hematoma formation (harder for subclavian approach)
Arterial cannulation: Has decreased due to ultrasound use but if you do cannulate an arterial site, don’t panic. Don’t remove the line. You can check a blood gas or arterial pulse waveform to confirm placement. Call vascular surgery for open removal and repair or endovascular repair. You could potentially remove a femoral arterial line and hold pressure but seek vascular advice regarding possible closure devices to use after removal.
Category: Critical Care
Keywords: Shock, hemodynamics, RIAD, Renal interlobar artery doppler, Resistive Index (PubMed Search)
Posted: 6/16/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
Renal Resuscitation using Renal Interlobar Artery Doppler (RIAD)
Shocked patient…. check! Adequate volume resuscitation…. check! Vasopressors.… check! Mean arterial pressure (MAP) > 65 mmHg….. check! Adequate urine output…. Wait, why isn’t my patient making urine?
As we begin to understand more about shock, hemodynamics, and the importance of perfusion over the usual macrocirculatory goals (MAP > 65), finding ways to assess regional blood flow is critical. A recent study examined the effect of fluid administration on renal perfusion using renal interlobar artery Doppler (RIAD) to assess the interlobar resistive index (RI). See how to perform a RIAD here.
They also recorded the fluid challenge’s effect on the traditional hemodynamic measurements of MAP and pulse pressure (PP) then observed the patient’s urine output (as a clinical marker of perfusion). The authors reported 3 key findings:
Bottom Line: The use of ultrasound to determine intrarenal hemodynamics is an interesting strategy to guide renal resuscitation in the shocked patient. There is mixed data on the use of RIAD, however this study could explain the findings of SEPSISPAM and also addresses the growing concern that traditional hemodynamic goals may be inadequate resuscitation targets.
References
For more critical care & resuscitation pearls, follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 6/9/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Intraosseous (IO) placement is a rapid and reliable method for obtaining venous access in critically ill patients; previous studies demonstrated that everything from vasopressors to packed RBCs can be infused through it.
This prospective observational study compared the first-pass success rate and time to successful placement of IO versus landmark-based (i.e., not ultrasound guided) central-line placement (femoral or subclavian access) during medical emergencies (e.g., cardiac arrest) in an inpatient population.
The first pass success rate for IO was found to be significantly higher than the landmark technique (90% vs. 38%) and placement was significantly faster for IOs (1.2 vs. 10.7 minutes).
Despite the fact that this study did not directly compare IO to ultrasound guided line placement, this study demonstrates that IO is a rapid and effective means to obtain central access during patients with emergent medical conditions.
Bottom-line: Consider placing an IO line when rapid central access is necessary.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: HFNC, high flow, vapotherm, nasal cannula, respiratory failure, non invasive ventilation (PubMed Search)
Posted: 6/2/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
High Flow Nasal Cannula (HFNC) in acute respiratory hypoxemia
The Trial:
Results:
Bottom line:
Consider using HFNC prior to or while deciding on intubation in patients with hypoxemic respiratory failure usually due to pneumonia
Category: Critical Care
Posted: 5/26/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Stress-Induced Cardiomyopathy
Boland TA, et al. Stress-induced cardiomyopathy. Crit Care Med 2015; 43:868-93.
Category: Critical Care
Keywords: Pulmonary Embolism (PubMed Search)
Posted: 5/19/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
Advances in Catheter-Directed Therapy for Acute PE - The PERFECT Registry
Earlier this month, initial results from the multicenter PERFECT registry (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) were released. In this study, 101 consecutive patients with massive or submassive PE were prospectively enrolled to receive early catheter-directed therapy.
Inclusion criteria:
Therapy provided:
Outcomes: Clinical success (stabilization of hemodynamics, improvement in pulmonary hypertension and/or right heart strain, and survival to discharge) was achieved in 86% of patients with massive PE and 97% of patients with submassive PE. There were no major procedure-related complications or major bleeding events.
Bottom Line: In patients with massive or submassive pulmonary embolism, there is growing evidence that early catheter-directed therapy may become first-line therapy for selected patients.
1. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results from a Prospective Multicenter Registry. Chest. 2015 (ePub April, 2015)
For more Critical Care pearls, follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 5/12/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
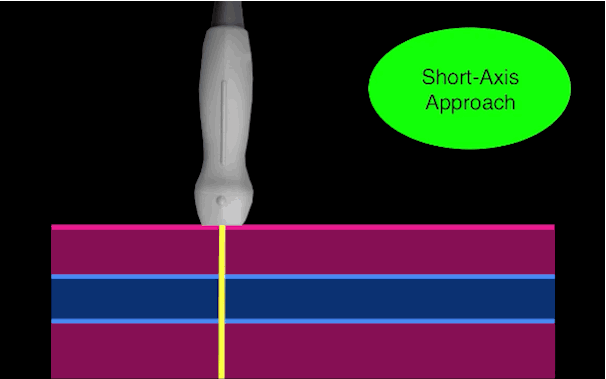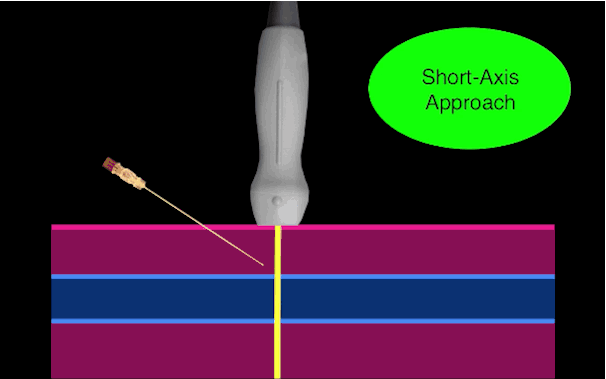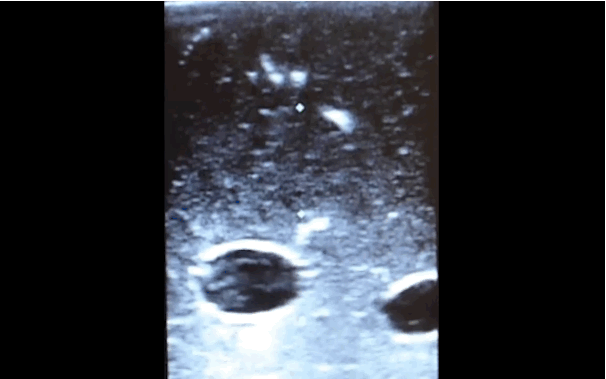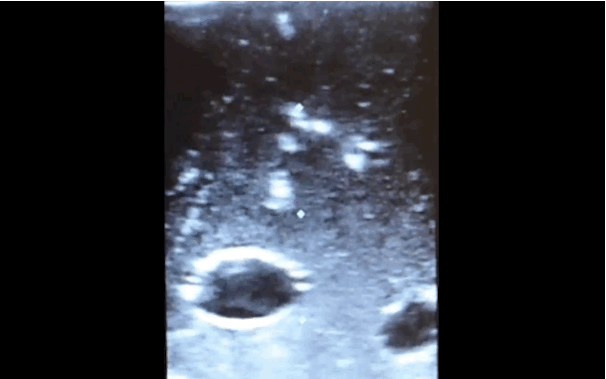
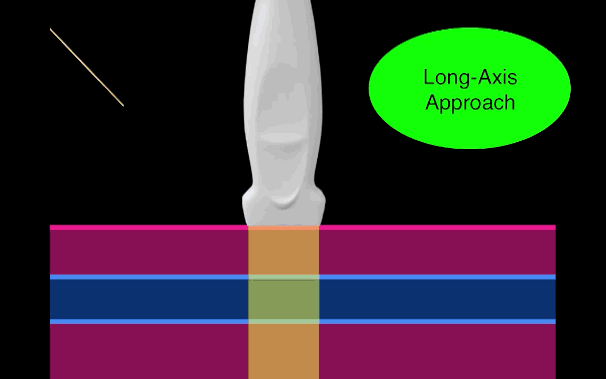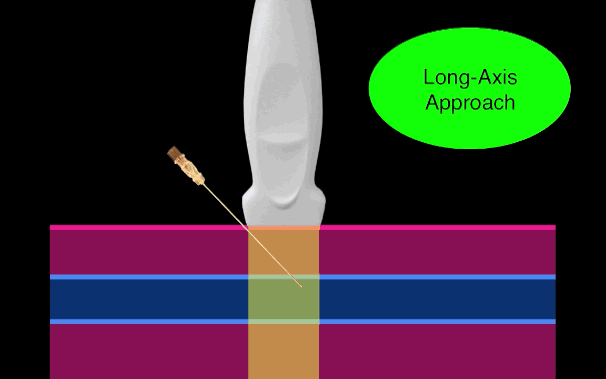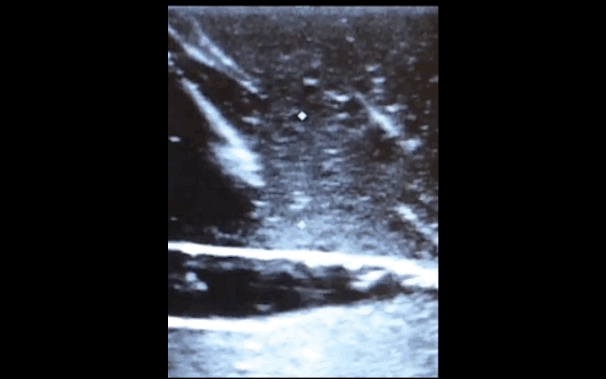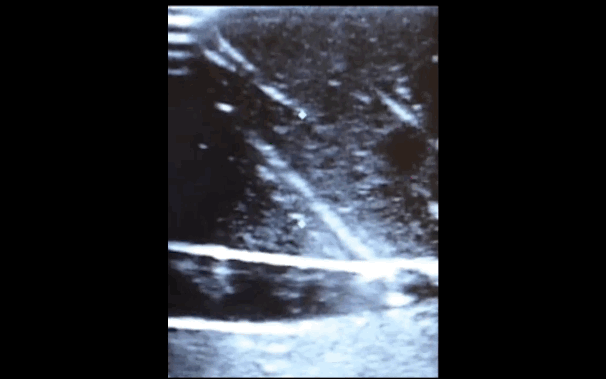
There is little debate that ultrasound-guided central lines are safer, faster, and more reliable compared to a landmark technique; there is some debate, however, as to whether the short axis (SA) or long axis (LA) approach is the best (see clips below).
The referenced study compared the SA and the LA technique for both the internal jugular (IJ) and subclavian (SC) venous approach. The authors measured number of skin breaks, number of needle redirections, and time to cannulation for each method.
This study demonstrated that the LA technique for subclavian placement had fewer redirections, decreased cannulation time, and fewer posterior wall punctures as compared to the SA. With respect to the IJ approach, the LA was also associated with fewer redirections than the SA view.
Bottom line: Consider the long-axis technique the next time you place an ultrasound guided central line.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: thoracentesis, pleural effusion, critical care (PubMed Search)
Posted: 5/4/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Safety of Thoracentesis
Results after 24 hours of followup post-procedure
Other interesting points:
Bottom line: Use your ultrasound to direct your tap and dont take out more than 1500 mL routinely
Ault MJ et al. Thoracentesis outcomes: a 12-year experience. Thorax 2015;70:127-132.
Category: Critical Care
Posted: 4/28/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
SIRS and Severe Sepsis Screening
Kaukonen KM, Bailey M, Pilcher D, et al. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis. NEJM 2015;372:1629-38.
Category: Critical Care
Keywords: large hemispheric infarct, acute ischemic infarct, stroke (PubMed Search)
Posted: 4/20/2015 by John Greenwood, MD
(Updated: 4/21/2015)
Click here to contact John Greenwood, MD
Updates in the Management of Large Hemispheric Infarction
Large hemispheric infarctions (LHI) are estimated to occur in 2-8% of all hospitalized ischemic strokes and 10 15% of all MCA territory infarcts. LHI carry high rates of morbidity and mortality, in fact, if left untreated associated cerebral edema can rapidly progress to transtentorial herniation and death in 40 80% of patients.
Recognized risk factors for progressive cerebral edema include:
Evidence based medical strategies for LHI include:
Prophylactic hemicraniectomy
Bottom Line: Early recognition of large hemispheric stroke is critical as it is associated with a high rate of morbidity and mortality. Aggressive medical management and early neurosurgical involvement may improve outcomes.
References
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 4/14/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
You decide to do a R.U.S.H. exam on your hypotensive patient and perform an apical four-chamber view.You see one of the two clips below; are there any tricks to figure out which is the left ventricle and which is the right ventricle?
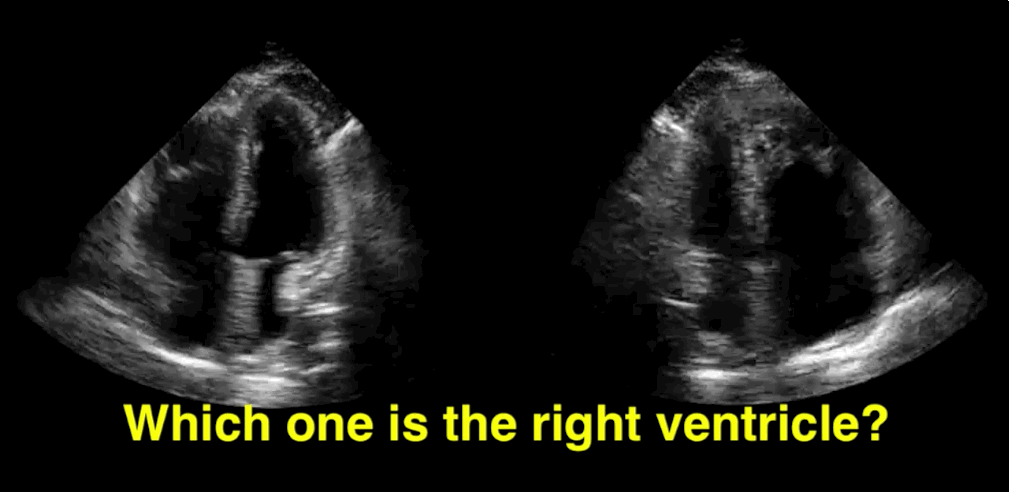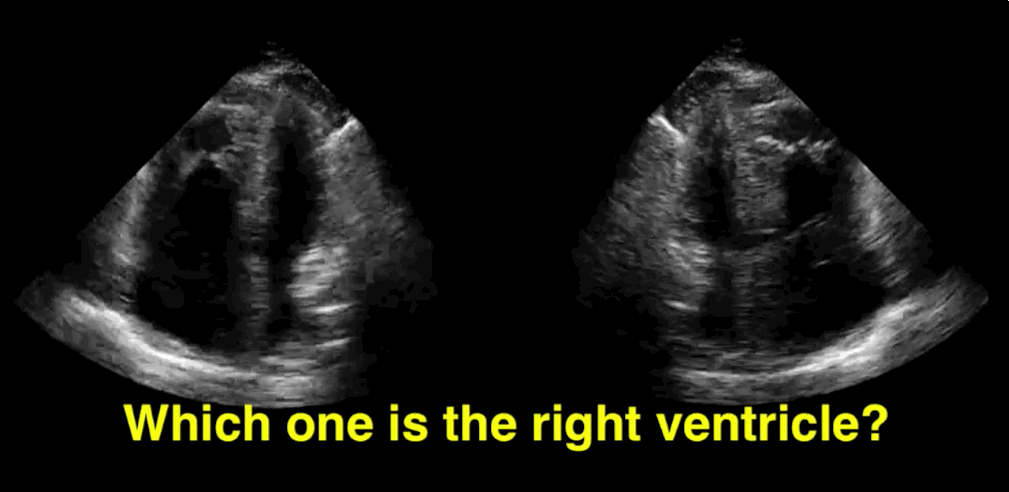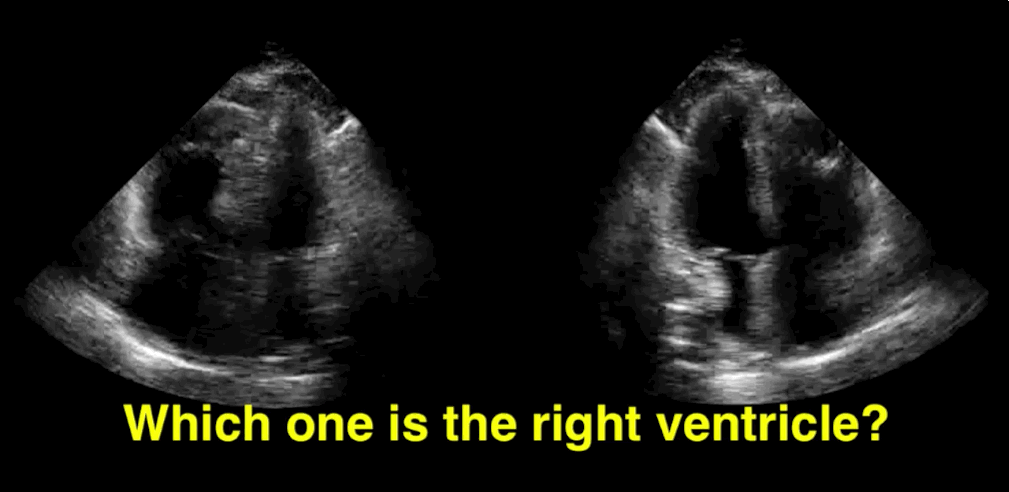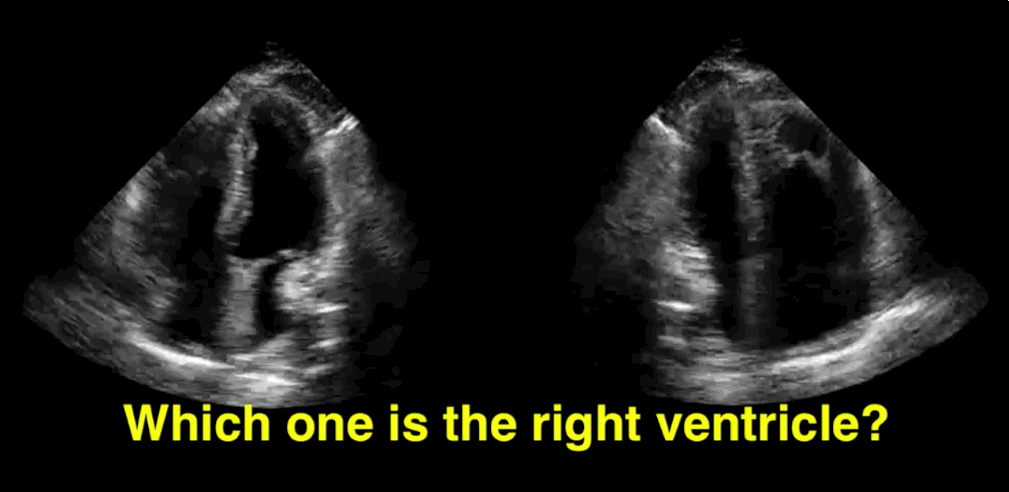
Tricks to distinguish the RV from LV in apical four-chamber view
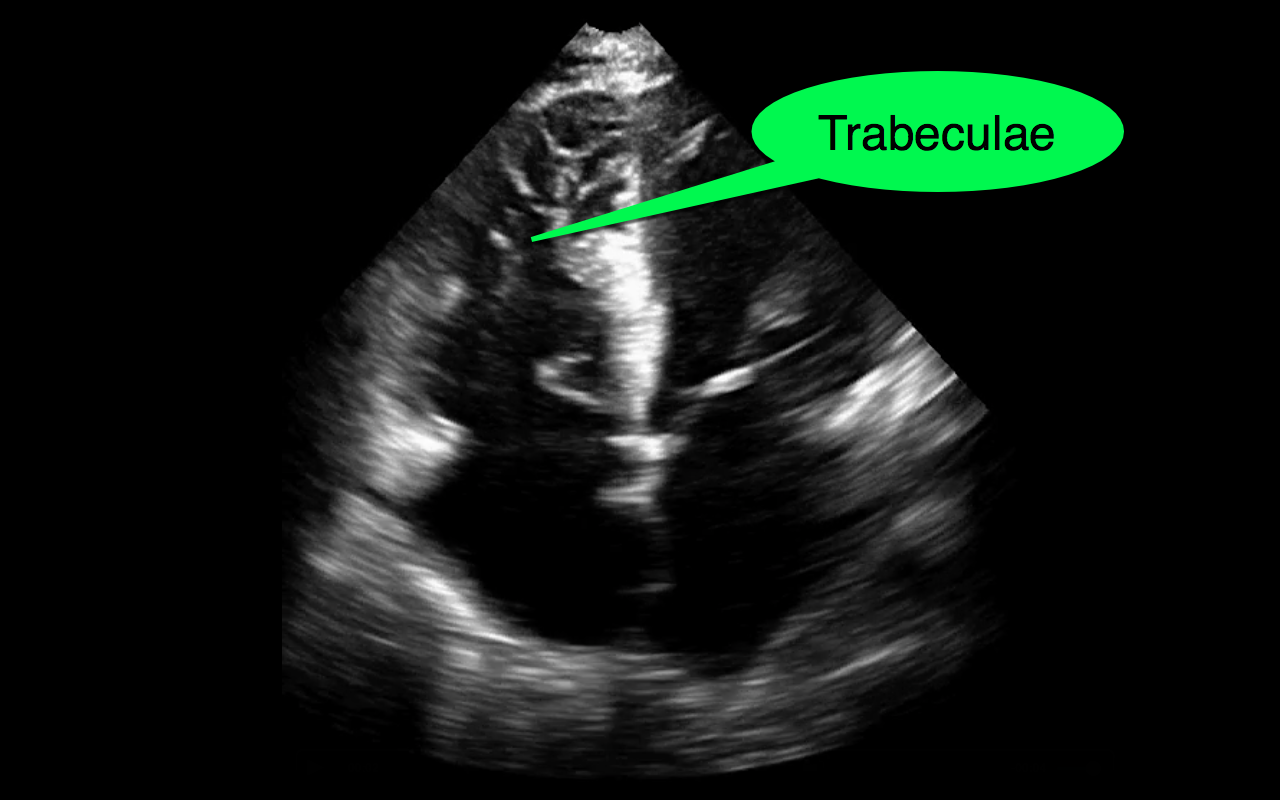
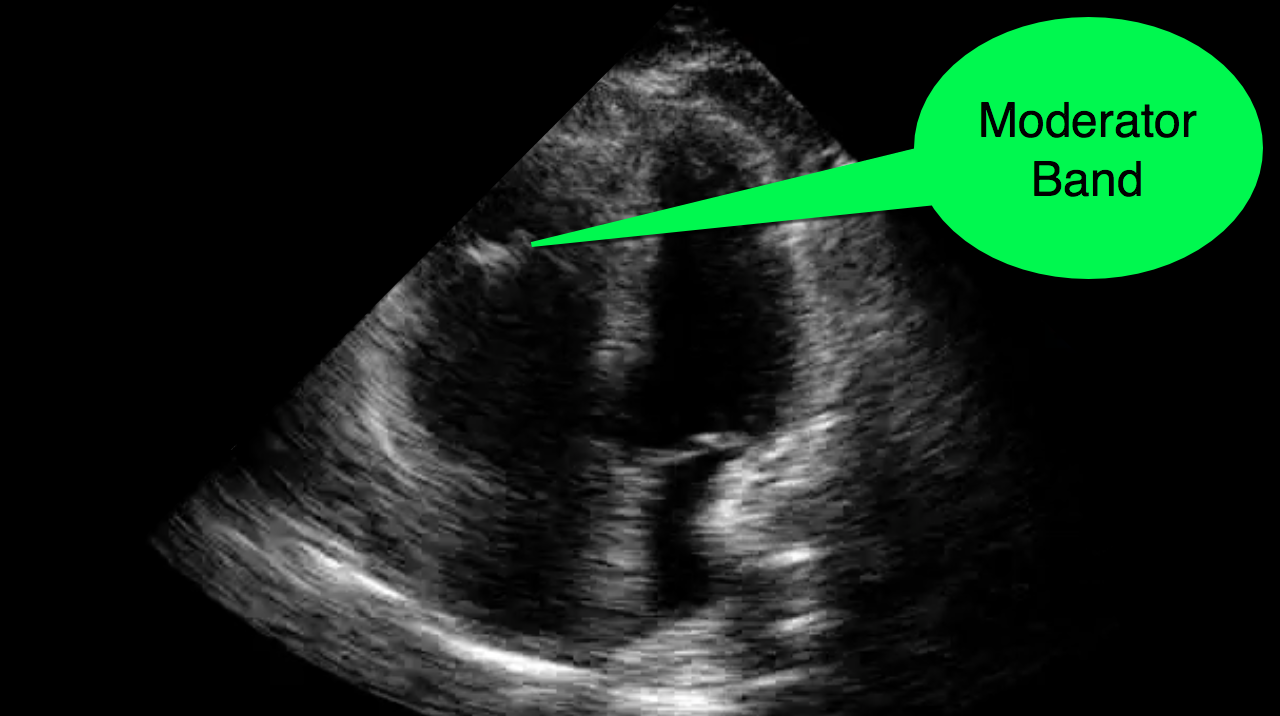
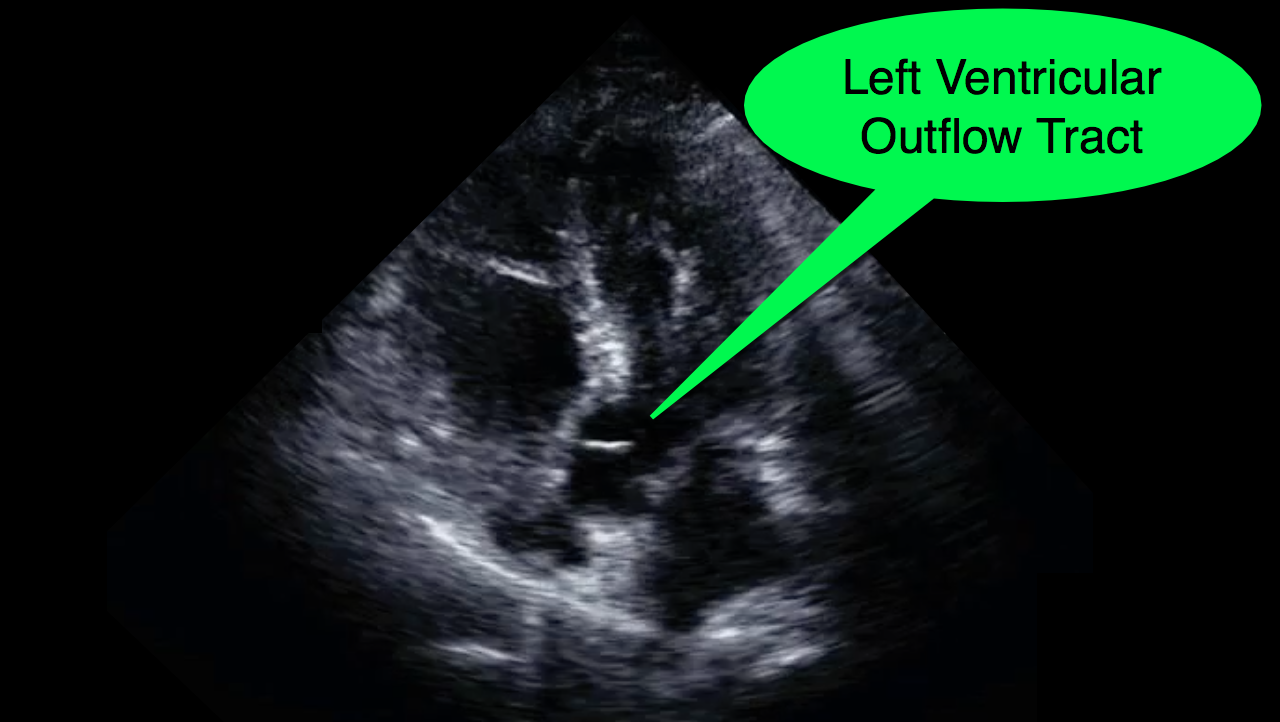
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: NIPPV (PubMed Search)
Posted: 4/7/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Cabrini L et al. Noninvasive ventilation and survival in acute care settings: a comprehensive systematic review and metaanalysis of randomized controlled trials. Crit Care Med 2015; 43:880-888.
Category: Critical Care
Posted: 3/31/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Mechanical Ventilation in the ED
Category: Critical Care
Keywords: mechanical ventilation, ARDS, PEEP (PubMed Search)
Posted: 3/24/2015 by John Greenwood, MD
(Updated: 2/1/2026)
Click here to contact John Greenwood, MD
Stop looking for the “Best PEEP”, aim for a “Better PEEP”
Mechanical ventilation settings in the patient with acute respiratory distress syndrome (ARDS) need to provide adequate gas exchange and prevent ventilator induced lung injury (VILI). Positive end-expiratory pressure (PEEP) is often prescribed with consideration of the patient’s FiO2 requirement, estimated chest wall compliance, and hemodynamic tolerance.
So what is the best strategy for PEEP prescription?
In a recent review, Gattinoni & colleagues analyzed a number of the recent studies examining PEEP optimization. In this paper, the authors conclude that there is no “Best PEEP,” and regardless of the level chosen there will be some degree of intratidal recruitment-derecruitment and VILI. They go on to recommend a PEEP prescription strategy that reflects the severity of ARDS using the patient’s PaO2/FiO2 or P/F ratio.
Bottom line: There is no “Best PEEP” however, a “Better PEEP” is one that is primarily tailored to the severity of the patient’s ARDS, but also compensates for chest wall resistance and minimizes hemodynamic compromise.
References
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 3/17/2015 by Haney Mallemat, MD
(Updated: 3/18/2015)
Click here to contact Haney Mallemat, MD
The results of a multi-center trial from the UK, the ProMISe trial, were just released and it confirms what two prior studies (i.e., ProCESS and ARISE) have already shown; there does not appear to be any difference in mortality when septic patients are treated with a strategy of early-goal directed therapy as compared to usual care.
Patients were included in the ProMISe trial if they were in septic shock and were then randomized to either the EGDT group (630 patients) or the usual care group (630 patients); a total of 1,260.
The primary end-point was all cause mortality at 90 days and there was no difference shown in the primary outcome. There were no differences found in the measured secondary outcomes (e.g., serious adverse events)
This trial adds to the evidence that septic patients may not benefit from protocolized (i.e., EGDT) care versus usual care. One explaination why, is that our "usual care" in 2015 has significantly changed since the introduction of EGDT in 2001.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
http://www.nejm.org/doi/pdf/10.1056/NEJMoa1500896
Category: Critical Care
Keywords: massive transfusion, trauma, bleeding, critical care, severe trauma, PROPPR (PubMed Search)
Posted: 3/10/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Transfusion in Major Trauma: The PROPPR Trial
What should we be transfusing in major trauma?
The Trial
Results
Conclusions
How does this affect my practice?
A 1:1:1 transfusion practice is safe and can decrease mortality from hemorrhage in major trauma
Other points: control bleeding, permissive hypotension, avoid crystalloids, use TEG to guide therapy (TXA etc)
Category: Critical Care
Posted: 3/3/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
High-Flow Nasal Cannula for Apneic Oxygenation
Miguel-Montanes R, et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med 2015;43:574-83.
Category: Critical Care
Keywords: CVP (PubMed Search)
Posted: 2/24/2015 by John Greenwood, MD
Click here to contact John Greenwood, MD
The Role of the CVP in a Post- “7 Mares” Era
The role for using central venous pressure (CVP) as a measure of volume responsiveness has largely fallen out of favor over the years.1 There are certainly better indices for fluid responsiveness, but don’t be fooled – the CVP isn’t a one trick pony. In fact, a high or rapidly rising CVP should raise a significant concern for impending cardiovascular collapse.
Consider the following differential diagnosis in the patient with an abnormally high or rising CVP ( >10 cm H2O).
Bottom Line: In a time where the utility of the CVP has been largely dismissed, remember that an abnormal CVP offers great deal of information beyond a simple measure of volume status.
References
Follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 2/17/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
As the cold and snow rips through the United States, hypothermia is a major concern because each year approximately 1,300 Americans die of hypothermia.
Classification of hypothermia:
The risk of cardiac arrest increases when the core temperature is less than 32 Celsius and significantly rises when the temperature is less than 28 Celsius. Rapid rewarming is required as part of resuscitation should cardiac arrest occur.
A rescue therapy to consider (when available) is extra corporeal membrane oxygenation (ECMO). ECMO not only provides circulatory support for patients in cardiac arrest, but allows re-warming of patients by 8-12 Celsius per hour.
Some studies quote survival rates of 50% with hypothermic cardiac arrest patients receiving ECMO versus 10% in similar patients who do not receive ECMO.
As winter lingers in the United States, consider speaking to your cardiac surgeons now to plan an Emergency Department protocol for hypothermic patients that may require ECMO.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
