Category: Critical Care
Keywords: VAP, chlorhexidine baths, subglottic suctioning (PubMed Search)
Posted: 2/10/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Updates in preventative strategies in the ICU
Preventing Ventilator Associated Pneumonia (VAP)
The trial
Bottom Line
Daily bathing with chlorhexidine does not reduce health care associated infections
The trial
Bottom Line
Category: Critical Care
Posted: 2/3/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
Hypertensive Emergency Pearls
Monnet X, Marik PE. What's new with hypertensive crisis? Intensive Care Med 2015; 41:127-130.
Category: Critical Care
Keywords: Methanol, toxicology, methanol toxicity, critical care (PubMed Search)
Posted: 1/20/2015 by John Greenwood, MD
(Updated: 1/30/2015)
Click here to contact John Greenwood, MD
Extracorporeal Treatment Strategies for Acute Methanol Poisoning (When to Dialyze)
Methanol toxicity is classically included in the differential for the intoxicated patient presenting to the ED. Add a negative EtOH level, anion/osmolar gap, blindness and you have yourself a slam dunk diagnosis. The goal is to stop the liver from metabolizing methanol to formic acid. Outside of fomepizole (or old school ethanol therapy), dialysis is often discussed, but when should you actually get the nephrologist on the phone?
This month the Extracorporeal Treatments in Poisoning Workgroup released a systematic review and consensus statement to help clinicians decide when to pull the HD trigger. Their suggestions are below.
When to start HD:
Which Modality: Intermittent HD (IHD) should be used over continuous renal replacement therapies (CRRT), as you can clear the toxin faster with higher HD flows.
When to stop HD: Extracorporeal treatment can be terminated when the methanol concentration is less than 200 mg/L or 6.2 mmol/L and a clinical improvement is observed.
Bottom Line: Consider early hemodialysis in most patients presenting with methanol toxicity. Clinical exam and routine lab testing will likely provide enough information to determine the need for IHD, but specific methanol levels can be helpful to guide adjunctive treatment options.
Reference
Roberts DM, Yates C, Megarbane B, et al. Recommendations for the Role of Extracorporeal Treatments in the Management of Acute Methanol Poisoning: A Systematic Review and Consensus Statement. Crit Care Med. 2015;43(2):461-472.
Follow me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 1/20/2015 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: diaphragm weakness, respiratory failure (PubMed Search)
Posted: 1/13/2015 by Feras Khan, MD
Click here to contact Feras Khan, MD
Diaphragm weakness and its significance
There are several ways to monitor diaphragm strength and function
Clinical Relevance
Curr Opin Crit Care. 2015 Feb;21(1):34-41. doi: 10.1097/MCC.0000000000000168.
Monitoring and preventing diaphragm injury.
Category: Critical Care
Posted: 1/6/2015 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
"PQRST" - Capnography in Cardiac Arrest
Heradstveit BE, Heltne JK. PQRST - A unique aide-memoire for capnography interpretation during cardiac arrest. Resuscitation 2014; 85:1619-20.
Category: Critical Care
Posted: 12/30/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Cartoons Kill: A new high-risk patient for critical illness & death
This past month, the BMJ published an impressive retrospective review that analyzed nearly 80 years of data to find that animated characters in children’s films are in fact at a very high-risk for death when compared to characters in adult dramas.
Films ranged from 1937 (Snow White) to 2013 (Frozen) and were compared against the two highest gossing dramatic films in that same year. The authors found that nearly two thirds of the children’s animated films contained an on-screen death of an important character compared to only half in adult dramas.
Fatalities were most commonly the result of:
Other high-risk animated characters include the parents of the protagonist (17.8% mortality) and nemeses (28.9% mortality). Median survival time was approximately 90 minutes (much less than the usual ED LOS!)
Notable early on-screen deaths included Nemo’s mother being eaten by a barracuda 4 minutes into Finding Nemo, Tarzan’s parents being killed by a leopard 4 minutes into Tarzan, and Cecil Gaines’ father being shot in front of him 6 minutes into The Butler.
The author’s intention was to point out the psychological impact of death on young children, but I think the authors also highlight an important, high-risk patient population that could present to your ED.
Bottom Line: Animated characters should be aggressively resuscitated and strongly considered for admission to a higher level of care should they present to your ED, as they appear to be at high-risk for death and rapid decompensation.
May all of you have a happy and safe 2015!
Reference
1. Colman I, Kingsbury M, Weeks M, et al. CARTOONS KILL: casualties in animated recreational theater in an objective observational new study of kids' introduction to loss of life. BMJ. 2014;349:g7184.
Follow me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 12/23/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Treating ischemic strokes with interventional therapies (e.g., clot retrievers, stents, intra-arterial tPA, etc.) is nothing new, but there has never been a randomized control trial demonstrating benefit until recently.
The prospective MR CLEAN trial evaluated whether interventional therapies (i.e., either mechanical intervention or intra-arterial tPA) would confer benefit; patients were included if there was an acute occlusion within the proximal intracranial portion of the anterior cerebral circulation.
90% of patients received alteplase prior to randomization; there were 233 patients in the intervention group (alteplase + intraarterial intervention) and 267 patients in the usual care care arm (alteplase only); all patients were treated within 6 hours of symptoms onset
The primary outcome was functional independence at 90 days; an absolute difference of 13.5 percentage points favoring the intervention group was found. There were no significant differences in mortality or symptomatic intracerebral hemorrhage.
Despite these exciting results, we must pause and ask why this was this the first randomized trial demonstrating benefit when previous trials could not? Here are three blogs posts that deep dive this question and raise even more questions:
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: influenza, tamiflu, (PubMed Search)
Posted: 12/16/2014 by Feras Khan, MD
Click here to contact Feras Khan, MD
How does it present?
Who cares…I got my vaccine! Does the vaccine work this year?
Can I test for this?
The CDC is recommending treatment...wait I thought we were done with Tamiflu?
Who is at risk/who deserves consideration for treatment?
Pearls of treatment
What are the side effects of anti-viral agents?
http://www.cdc.gov/flu/index.htm
Category: Critical Care
Posted: 12/9/2014 by Mike Winters, MBA, MD
Click here to contact Mike Winters, MBA, MD
The Critically Ill Patient with Ebola Virus Disease
Fowler RA, et al. Caring for Critically Ill Patients with Ebola Virus Disease. Am J Resp Crit Care Med 2014; 190:733-37.
Category: Critical Care
Posted: 12/2/2014 by John Greenwood, MD
Click here to contact John Greenwood, MD
Dynamic Measures of Intravascular Volume Assessment
The resuscitation of a patient in shock often requires the administration of intravenous fluid. Excessive fluid resuscitation can lead to worsening pulmonary edema, systemic edema, acid-base disturbances, as well as many other complications. There are a myriad of techniques to try and figure out if the patient needs more intravascular volume, but each has it’s pitfalls.
Recently, experts have recommend that we move away from using static measures of preload assessment such as central venous pressure (CVP) and instead focus on using dynamic measures for volume responsiveness.
Volume Responsiveness Defined: An increase of stroke volume of 10-15% after a 500 mL IV crystalloid bolus over 10-15 minutes.
Below is a chart describing key values, requirements, and contraindications for each of these dynamic measures of non-invasive intravascular volume assessment.
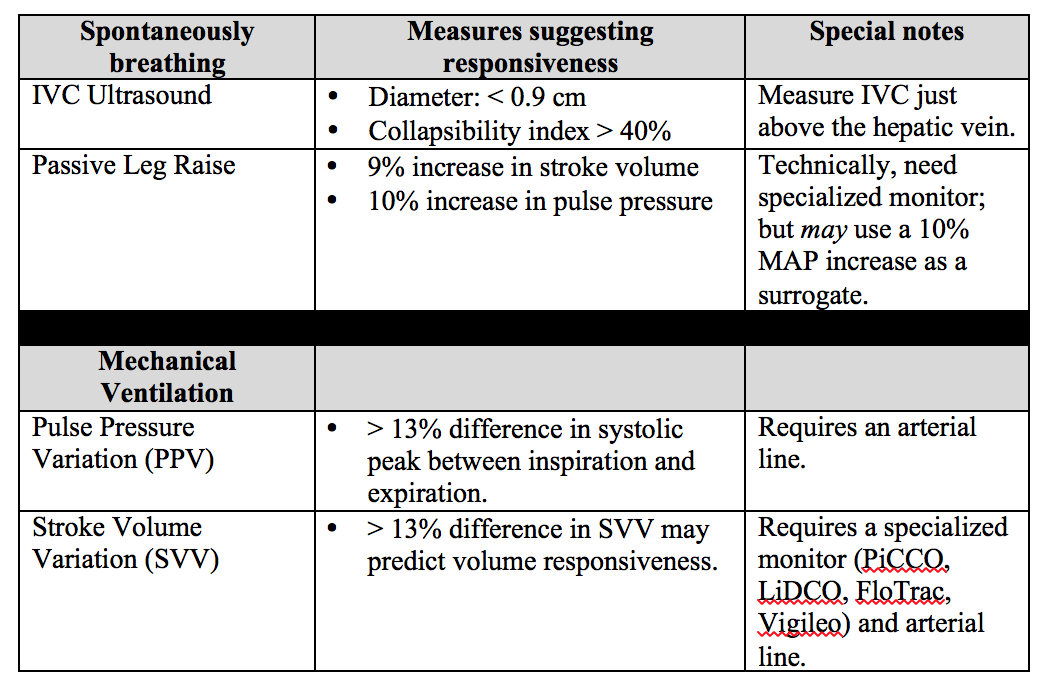
Important notes: PPV and SVV require the patient to be intubated with controlled tidal volumes. Arrhythmias and right heart failure make many of these measures invalid (except for PLR). Other methods of assessment not discussed include systolic pressure variation, left ventricular outflow track velocity time integral (LVOT VTI), and end-expiratory occlusion pressure (EEO).
Bottom Line: None of these measures are perfect and shouldn't be used in isolation to determine if the patient’s “tank is full”. Combine clinical judgment with these measures to get a best estimate of whether or not to give that next fluid bolus.
Reference
1. Enomoto TM, Harder L. Dynamic indices of preload. Crit Care Clin. 2010;26(2):307-21,
Follow Me on Twitter @JohnGreenwoodMD
Category: Critical Care
Posted: 11/25/2014 by Haney Mallemat, MD
Click here to contact Haney Mallemat, MD
Running a successful resuscitation not only means doing everything right, but also remembering all the things that can go wrong. A.E.I.O.U. is a simple mnemonic that can help you remember the simple things that are sometimes forgotten during a medical resuscitation.
A – Advanced airway equipment to bedside, as well as checking the correct placement of the Airway if a patient is intubated in the field. Also consider adding another A, for Arterial line; early placement can help with pulse checks and an accurate assessment of blood pressure should there be return of spontaneous circulation (ROSC); the femoral site is fast and accurate.
E – End-tidal CO2 (ETCO2) helps detect ROSC. Ask for the ETCO2 monitor to be set up right after you receive notification of an arrest in transit; ETCO2 requires time to set-up / calibrate
I – Intraosseous line(s); compared to peripheral or central venous access, IO’s are faster, safer, and any medication can be administered through it, including vasopressors / inotropes.
O – Order (i.e., “who’s who in the Resus room?); You may be the team leader or you may be assisting, but it is important that you, and everyone else in the room, know their role prior patient arrival. If you are leading the resus, be sure everyone knows who you are, and assign everyone in the room a specific task (e.g., chest compressions, IO placement, etc.). If you are assisting and have not been assigned a task, ask the resus leader what you can do to help. If there is nothing immediate for you to do then take the initiative to de-clutter the room and step outside; be nearby and ready to help, if needed.
U – Ultrasound; can help prognosticate and detect reversible causes (e.g., pericardial tamponade). Have the ultrasound machine in the room prior to patient arrival. It should be powered on, with the proper probe connected, and in the proper mode. The most experienced ultrasonographer should scan the patient during a pulse check; experience is vital because hands-off time should be minimized.
*Tips for the Resuscitationist (#TFTR) is a new series to help you to better manage your critically ill patients. Do you have an idea for a topic or do you have a tip you would like to share? Send it to us via twitter @criticalcarenow (use (#TFTR)). You can also email us here.
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)
Category: Critical Care
Keywords: cap, pneumonia, (PubMed Search)
Posted: 11/18/2014 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
Tips for the inpatient management of community acquired pneumonia
Daniel M. Musher, M.D., and Anna R. Thorner, M.D.
N Engl J Med 2014; 371:1619-1628October 23, 2014DOI: 10.1056/NEJMra1312885
Category: Critical Care
Posted: 11/11/2014 by Mike Winters, MBA, MD
(Updated: 11/12/2014)
Click here to contact Mike Winters, MBA, MD
Aminoglycosides in Critically Ill Patients
Matthaiou DK, et al. What is new in the use of aminoglycosides in critically ill patients? Intensive Care Med 2014; 40:1553-1555.
Category: Critical Care
Keywords: Airway, critical care, RSI, rapid sequence intubation (PubMed Search)
Posted: 11/3/2014 by John Greenwood, MD
(Updated: 11/4/2014)
Click here to contact John Greenwood, MD
Back 2 Basics Series: Your Simple RSI Checklist - SOAP ME
The use of a checklist during high stress medical procedures is often recommended. Rapid sequence intubation (RSI) is a classic situation where having a checklist can ensure adequate preparation however, if you don’t have a checklist – this simple mnemonic will make sure you are well prepared for a successful intubation.
| Mnemonic – “SOAP ME” | |
|---|---|
| Suction |
|
| Oxygen |
|
| Airways |
|
| Positioning |
|
| Monitors & Meds |
|
| EtCO2 & other Equipment |
|
The SOAP ME mnemonic is a quick and useful technique to remember only the basics of airway management and preparation. Always remember to also assign roles to team members and communicate clearly to maximize your chances of success.
Category: Critical Care
Keywords: choosing wisely, icu, critical care (PubMed Search)
Posted: 10/21/2014 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
Choosing Wisely in the ICU
The Critical Care Societies Collaborative came up with this list for ICU providers
1. Don’t order diagnostic tests at regular intervals (such as every day) but rather in response to specific clinical questions. Do you really need a daily INR check or CBC check in all ICU patients? Really?
2. Don’t transfuse red blood cells in hemodynamically stable, non-bleeding ICU patients with a hemoglobin concentration greater than 7 g/dl. See last week’s Pearl!
3. Don’t use parental nutrition in adequately nourished critically ill patients within the first seven days of an ICU stay. TPN is the Cinnamon Toast Crunch of fungi.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation. Use as little as possible when you can.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort. Engage families early in the hospital stay regarding aggressive life-sustaining treatments. Get palliative care involved in the ED!
Crit Care Med. 2014 Nov;42(11):2437-8. doi: 10.1097/CCM.0000000000000696.
Angus DC1, Deutschman CS, Hall JB, Wilson KC, Munro CL, Hill NS.
Category: Critical Care
Posted: 10/14/2014 by Mike Winters, MBA, MD
(Updated: 2/1/2026)
Click here to contact Mike Winters, MBA, MD
Hemoglobin Threshold in Septic Shock
Holst LB, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. NEJM 2014; [published online]
Category: Critical Care
Posted: 10/6/2014 by John Greenwood, MD
(Updated: 10/7/2014)
Click here to contact John Greenwood, MD
The ARISE Trial
Early, aggressive resuscitation and attention to detail are essential element of managing critically ill patients. This past week the ARISE trial was published - a 2nd large, randomized control study to examine the benefit of protocolized vs. usual care in patients with severe sepsis and septic shock.
What were the main findings? After enrolling 1,600 patients who presented to the ED in severe sepsis or septic shock:

Bottom Line: Resuscitation goals for the patient with septic shock should include:
Additional therapeutic goals should be made on a patient by patient basis. Reassess your patient frequently, pay attention to the details, and you will improve your patient’s mortality.
Suggested Reading
Follow Me on Twitter: @JohnGreenwoodMD
Category: Critical Care
Posted: 9/30/2014 by Haney Mallemat, MD
(Updated: 10/1/2014)
Click here to contact Haney Mallemat, MD
The last Back to the Basics post discussed the use of vasopressors to improve hemodynamics by increasing arterial (and venous) tone. This time we’ll discuss the use of agents to increase inotropy for patients with severe systolic dysfunction / failure.
Dobutamine: a direct b1 and b2-receptors agonist. It has no peripheral vasoconstrictor properties, so if blood pressure increases it occurs secondary to increased cardiac output. Unfortunately, blood pressure may be decreased in some patients due to its peripheral vasodilatory effects; in these cases it may need to be used with a vasopressor.
Milrinone: augments contractility by increasing intracellular Ca levels via cellular phosphodiesterase inhibition. Because it does not work on beta-receptors, it might be preferred for patients taking beta-blockers requiring inotropic support. It may cause peripheral vasodilation and hypotension, but this may be a benefit if pulmonary artery pressure is elevated as reductions in pulmonary artery pressure lead to improvements in right ventricular function. It has a long-half life and should be avoided in patients with renal impairment.
Dopamine: chemical precursor to norepinephrine and technically a vasopressor. At moderate doses (3-10 mcg/kg/min) it works on beta-receptors to increase myocyte contractility. At higher doses works primarily as a vasopressor, which may reduce cardiac output due to higher afterload.
Norepinephrine/epinephrine: has alpha and beta properties that lead to increased peripheral vasoconstriction, but also increases inotropy and chronotropy (faster heart rate)
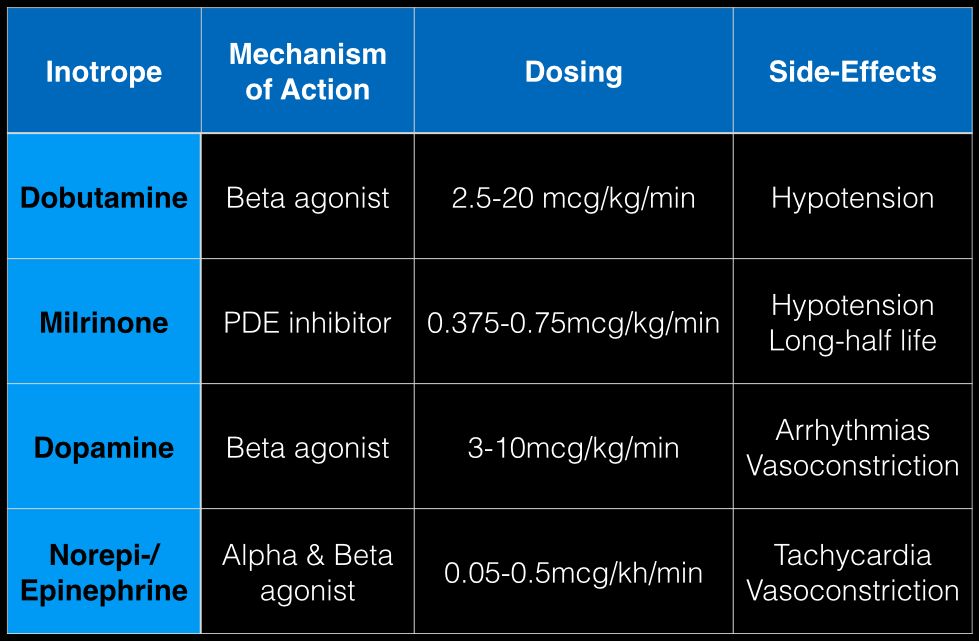
Follow me on Twitter (@criticalcarenow) or Google+ (+criticalcarenow)a
Category: Critical Care
Keywords: massive transfusion, bleeding (PubMed Search)
Posted: 9/23/2014 by Feras Khan, MD
(Updated: 2/1/2026)
Click here to contact Feras Khan, MD
What is a massive transfusion?
When would I use this?
Indications:
-Systolic Blood pressure < 100
-Unable to obtain blood pressure
AND
-Penetrating torso trauma
-Positive FAST
-External blood loss
-Plans to go to the OR
How do I give it?
Does this apply for just traumatic bleeding?
Are there other agents I can use?
What am I trying to do with this protocol?
Murthi SB, Stansbury LG, Dutton RP, et al. TRAnsfusion medicine in trauma patients: an update. Expert Rev Hematol. 2011 Oct;4(5):527-37.
Hess JR, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008 Oct; 65(4):748-54.
University of Maryland SHOCK Trauma Massive Transfusion Protocol. 2011.
